
Frameless, real-time, surface imaging-guided radiosurgery: update on clinical outcomes for brain metastases
Introduction
Stereotactic radiosurgery (SRS) is a non-invasive way to target a small area inside the body using relatively high doses of radiation. One common and important use of SRS is its ability to treat intracranial metastatic tumors. It has been shown in previous studies that local tumor control can be achieved by SRS alone or SRS alongside whole brain radiation therapy (WBRT) (1-4).
For earlier SRS delivery, a head frame was used to prevent patient movement. These head frames required rigid, screw fixation into the patient’s skull. However, certain disadvantages such as fractionation limitation as well as discomfort were troublesome (5). Lately, newer frameless image guided SRS systems, which provide similar accuracy to that of head frame-based therapy, have been popularized (6-8). The newer systems use a customized plastic mask in place of a head frame. But like the frame-based models, the frameless models also have disadvantages. Since patients are exposed to CT scans and serial X-rays, infrared fiducials are required in order to abide by radiation regulations which limit the amount of ionizing radiation exposure a patient receives (9,10). In some systems, the fiducials are attached to a bite block (Figure 1A), and the bite block, not the patient per se, is subsequently tracked during the procedure. Patients with poor dental structure or those with an inability to breath freely through their nose were difficult, if not impossible, to treat. Another noted drawback is the possibility of fiducial movement at the attachment point (11).
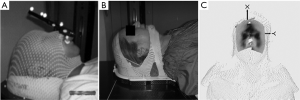
To avoid these drawbacks, surface image guided (SIG) systems are being used to deliver SRS. One such system is AlignRT (VisionRT Ltd, London, England), which uses three ceiling mounted non-ionizing camera pods. Accuracy of AlignRT is similar to that of cone-beam CT (CBCT) and infrared marker tracking (12-14). The AlignRT system provides real-time monitoring of facial landmarks during treatment. In this report, we will present our updated clinical outcomes of patients with brain metastases that were treated with real-time, frameless, non-ionizing SIG radiosurgery (SIG-RS).
Patients and methods
Patient and treatment characteristics
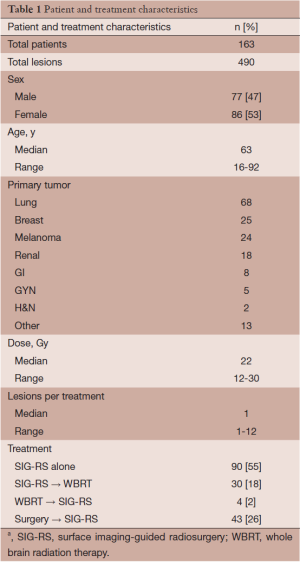
Medical records for 163 patients with a minimum age of 16 were retrieved for this study, with approval from the local institutional review board. A total of 490 lesions were reported between December 2008 and April 2014 among the 163 patients. Forty-three of the patients (26%) presented with a total of 45 cavity resections. Ninety patients were treated with SRS alone. Forty-three patients (26.4%) received surgical resection followed by SIG-RS. Thirty patients (18%) received WBRT following SIG-RS and four patients (2.4%) received WBRT prior to SIG-RS. Patients received a median dose of 22 Gy (range, 12-30 Gy) when treated with SIG-RS. Patient and treatment characteristics are further noted in Table 1.
Full table
Real-time, frameless SIG-RS
Patients were analyzed by magnetic resonance imaging (MRI) with 1.25-mm axial slice intervals using a 3.0-T MRI (General Electric, Fairfield, Connecticut). Patients were immobilized in two ways. Initially, a foam head mold (CDR Systems, Calgary, Alberta, Canada) was fit to the patient’s head for the first 44 patients. This setup proved to be inefficient as it required restraints and tape for stabilizing the patient’s forehead and jaw. For subsequent patients, a custom open-faced mask (CIVCO, Medical Solutions, Kalona, Iowa) was used and proved to be more efficient compared to the initial method as it provided stability to the forehead and mandible without the use of tape and restraints. In addition, an SRS headrest including a custom foam cushion (AccuForm, CIVCO Medical Solutions) was added to the open-faced mask for increased patient comfort and reproducibility. The open-faced mask can be seen in Figure 1B.
During simulation, patients with either the foam mold or open-faced mask were placed in a supine position. Simulation was done using a non-contrast CT (35-cm field of view, 512 × 512 pixel size, 1.25-mm slice interval). The CT and MRI were registered using the rigid registration in Varian Eclipse. Varian Eclipse software, version 8.9 (Varian Medical Systems, Palo Alto, California) was used for treatment planning. Gross tumor volume (GTV) and critical structures (brainstem, optic chiasm, optic nerves, normal brain, cochlea, eyes, and eye lenses) were contoured using MR guidance, and an isotropic 1 mm margin was added to the GTV to produce the planning target volume (15). A body contour was generated on the planning CT using Hounsfield unit (HU) cutoff of –700 HU. The CT body contour and plan isocenter was then sent to the surface imaging system, and a region of interest (ROI) to be used for surface monitoring was designated. The ROI includes the forehead, nose, zygomatic bones and temporal bones, but excludes the tip of nose and eye lids (Figure 1C). Treatment plans utilized either volume modulated arc therapy (VMAT) (RapidArc, Varian Medical Systems, Palo Alto, CA) or static field intensity modulated radiation therapy (IMRT), delivering several noncoplanar fields or arcs. Patients who presented with multiple lesions received radiation to all their targets concomitantly with a single isocenter.
On treatment day, patients were initially setup using the surface imaging system, AlignRT, with the planning CT generated surface as the target. The AlignRT system uses three camera pods; each pod contains three cameras (two for stereovision, one for texture). Each pod also contains a speckle flash unit for static imaging and a speckle projector for dynamic imaging. The AlignRT registration algorithm identifies errors with six degrees of freedom—vertical, lateral, longitudinal, roll, yaw, and pitch. After initial setup by AlignRT, orthogonal kV and CBCT imaging were performed to determine any necessary shifts. After any necessary shifts were applied, a new reference surface was acquired by AlignRT for intrafraction monitoring. On the day of treatment, patients were tracked using visual monitors at the control room. AlignRT monitored the patient’s in real-time (about five frames per second) throughout the duration of treatment. Predetermined translational threshold was between 1 and 2 mm and rotational threshold was 1°. Automatic Trilogy beam or manual TrueBeam hold was used if patient movement surpassed these thresholds. During beam hold, patients usually moved back into position without external intervention. If patients had difficulty reverting to a position, AlignRT guidance was used to reposition the patient.
Follow-up and statistical analysis
One hundred and thirty four of the 163 patients received follow up imaging studies after treatment. The remaining 29 patients either passed away before their scheduled MRI or imaging wasn’t available. Follow up times (the time difference between time of treatment and follow up MRI or death) were calculated for each patient. For those patients whose imaging was not available, they were not included in the local control analyses but all 163 patients were included in overall survival analysis. Local control, defined as a lack of progression of disease, was determined per patient and per lesion. Disease progression was defined as radiographic increase >20% of the sum of the largest diameters of a treated lesion. Additional imaging studies including MRI with spectroscopy, perfusion studies or brain PET/CT were also used in some of the cases to evaluate for recurrence vs. radionecrosis. Those patients whose disease showed recurrent local failure or new disease were subsequently treated with SIG-RS, WBRT or surgery. Analyses for local control and overall survival were calculated using the Kaplan-Meier method, and Kaplan-Meier curve differences were then analyzed using the log-rank test. SAS Statistics software provided all statistical analyses.
Results
Median follow-up for all patients was 6.7 months (range, 0.5-45.1 months); 119 of 163 patients (73%) deceased during the follow-up period. Median follow-up for the 44 surviving patients at the time of this analysis was 13.8 months (range, 1.4-47.6 months). Twenty-nine patients were excluded from the local control analysis because patients either died before repeat imaging studies to evaluate for treatment response or were lost to follow-up and there was no record. Six of the 29 excluded patients were lost to follow-up or there was no available record. Twenty-three patients of the 29 excluded patients died before repeat imaging studies with the median survival of 2.7 months (range, 0.07-8.5 months) from the time of treatment. Some of these patients entered into hospice and thus did not have any additional imaging studies after treatment. Follow-up imaging studies after treatment were available for the remaining 134 patients (82%) and consisted of contrast-enhanced MRI (96%) and CT (4%).
Local control
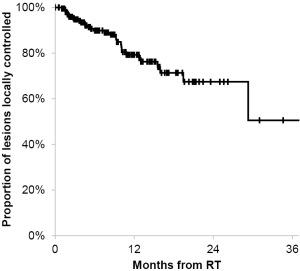
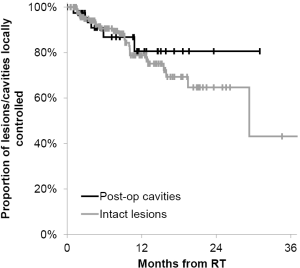
Of the 134 patients with available follow-up imaging studies, eighty-nine, thirty, twelve, three patients were treated once, twice, three times or four times at different time points, respectively for new lesions. Of the 134 patients, 26 patients (28 lesions and five post-operative cavities) had local failures as evidence on repeat imaging. The patients who had local failure either had repeat SIG-RS or whole brain radiotherapy or underwent surgical resection (three patients) and had pathological confirmation of viable tumors. Median time to local failure of individual brain metastases and/or post-operative cavities was 5.8 months (range, 1.0-29.3 months) from the time of treatment. The actuarial 6- and 12-month local control of treated lesions or post-operative cavities was 90% [95% confidence interval (CI), 84-94%] and 79% (95% CI, 71-86%), respectively (Figure 2). Patient demographics such as patient age, number of lesions, cavities, and primary tumor type were not associated with significant differences in local control. In addition, when local control was analyzed for post-operative cavities and intracranial intact lesions, there was also no statistically significant difference (Figure 3). The 12-month actuarial local control for post-operative cavities and intact lesions was 81% and 79%, respectively. SIG-RS was performed on 89 patients with one treatment and 45 patients with more than one treatment (range, 2-4 treatments).
Survival
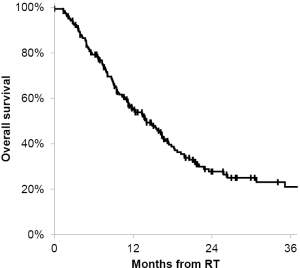
The median overall survival was 14 months from the time of the treatment. The actuarial 6- and 12-month overall survival for all patients was 80% (95% CI, 74-85%) and 56% (95% CI, 49-63%), respectively (Figure 4). There was no prognostic factors identified that affect overall survival.
Treatment times
As previous reported, the median treatment time from initial beam on to final beam off was 15 minutes. At our institute, SIG-RS was performed on either a TrueBeam LINAC or a Trilogy LINAC. The average treatment time was significantly shorter on the TrueBeam LINAC compared to that on the Trilogy LINAC. Since our previous report, more patients were being treated with SIG-RS on the TrueBeam LINAC to improve the work flow and machine time.
Discussion
Treatments for brain metastases include surgery, WBRT or SRS, or a combination of the above. At our institution, surgery is usually reserved for single large symptomatic lesion that are located in surgically accessible locations Whole brain radiotherapy remains the standard option for treatment of brain metastases, although this technique is used less and less at our center due to the known deleterious effects in subsequent neurocognitive function (16). SRS for brain metastases has emerged as an alternative treatment for brain metastases, either as standalone therapy or in conjunction with WBRT. Several randomized trials and multi-institutional studies have shown that SRS provides highly effective local tumor control (1-4). While local control is the primary goal of SRS for brain metastases, improvement in treatment techniques, especially with LINAC-based SRS, has advanced tremendously in the past few decades. Maximizing patient comfort during treatment, while maintaining the treatment accuracy and local control, has been the focus of the new treatment technique SIG-RS. SIG-RS accomplishes this goal by providing rigorous immobilization with real-time surface-imaging guidance for treatment accuracy without the use of an invasive head frame, a closed mask, or bite-block-based system. The efficient delivery of image-guided radiosurgical treatment is achieved without detriment to the accuracy of treatment, treatment time, or clinical outcomes. Feasibility studies utilizing surface-guided imaging SRS have been previously published (12-14) including our clinical experience and outcomes of SIG-RS in 44 patients with brain metastases (115 treated lesions) treated with this novel technique (17).
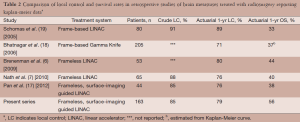
In this study, we provide an update of our experience and clinical outcomes of 163 patients totaling 490 lesions and 45 post-operative cavities treated with SIG-RS. Consistent with our previous report, this study shows a 6- and 12-month actuarial local control rate of was 90% and 79%, respectively. These results are comparable to previous frame-based (18,19) and frameless (6,7) studies reporting Kaplan-Meier data showing 12-month local control rates of 71% to 89% and 76% to 80%, respectively (Table 2). In this report, the majority of patients received SIG-RS alone with four patients receiving WBRT prior to SIG-RS and 30 patients received WBRT after SIG-RS due to new lesions detected on follow-up imaging studies. Overall survival was also analyzed as an additional end point. The majority of patients, 119 of 163 patients (73%), had deceased at the time of analysis. The 12-month actuarial overall survival in this study was 56%, which is also in line with results from previously published studies of 23-54% survival for patients treated with frame-based or frameless SRS methods (Table 2). We also found no significant statistical difference in local control in SIG-RS to post-operative cavities or intact brain lesions. It is likely due to the small number of post-operative cavities treated compared to a much larger number of intact lesions treated.
Full table
Although there was no objective documentation of patient comfort during the SIG-RS treatment in this study, we observed that it was very uncommon for patients to require Ativan for anxiety and some were comfortable enough to fall as sleep during treatment. Furthermore, patients did not report any discomfort with the frameless, bite-block-less set up. An additional benefit of SIG set up is that it provides non-ionizing, real-time monitoring without dependence on the reproducibility of the infrared bite block.
Equally important is the median treatment time with SIG-RS which compares favorably to other reports of LINAC-based SRS treatment (20,21). In the high dose rate delivery mode (1,400 MU/min), typical treatment beam-on times have been reduced to the point that scheduling SIG-RS patients now occurs during a routine 15 min time slot. This time period accounts for the surface imaging setup time, the CBCT setup verification time and beam-on time. This study extends our previous findings of local control in patients with brain metastases treated with SIG-RG at our institution. With a larger number of patients and number of lesions treated, the local control remained consistent and comparable to other published studies. However, this study has several important limitations inherent to retrospective study designs, including bias in determining local failures, patient selection, and missing or incomplete follow-up. Patients treated with SIG-RS who did not have follow-up imaging studies after the treatment were excluded from analysis of local control due to lack of data.
Conclusions
The results of this study confirm and extend our previous findings that SIG-RS for treating brain metastases can produce clinical outcomes comparable to those for conventional frame-based and frameless SRS techniques. At the same time, SIG-RS setup provides better comfort with an open-faced mask, and allows continuous non-ionizing tracking during the treatment delivery time. We also experienced improvements in workflow using the SIG-RS procedure as the simulations, setup, and treatment are very similar to other radiotherapy treatments. Experience with SIG-RS continues to grow at our institution, and is now applied routinely to all adult and pediatric cranial radiosurgery cases.
Acknowledgments
Funding: None. The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “SBRT/SRS in Radiation Research”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.07.08). The series “SBRT/SRS in Radiation Research” was commissioned by the editorial office without any funding or sponsorship. KTM served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Translational Cancer Research. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665-72. [PubMed]
- Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 2006;295:2483-91. [PubMed]
- Kondziolka D, Patel A, Lunsford LD, et al. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys 1999;45:427-34. [PubMed]
- Sneed PK, Suh JH, Goetsch SJ, et al. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int J Radiat Oncol Biol Phys 2002;53:519-26. [PubMed]
- Yeung D, Palta J, Fontanesi J, et al. Systematic analysis of errors in target localization and treatment delivery in stereotactic radiosurgery (SRS). Int J Radiat Oncol Biol Phys 1994;28:493-8. [PubMed]
- Breneman JC, Steinmetz R, Smith A, et al. Frameless image-guided intracranial stereotactic radiosurgery: clinical outcomes for brain metastases. Int J Radiat Oncol Biol Phys 2009;74:702-6. [PubMed]
- Nath SK, Lawson JD, Wang JZ, et al. Optically-guided frameless linac-based radiosurgery for brain metastases: clinical experience. J Neurooncol 2010;97:67-72. [PubMed]
- Tagaste B, Riboldi M, Spadea MF, et al. Comparison between infrared optical and stereoscopic X-ray technologies for patient setup in image guided stereotactic radiotherapy. Int J Radiat Oncol Biol Phys 2012;82:1706-14. [PubMed]
- Kearns WT, Urbanic JJ, Hampton CJ, et al. Radiation safety issues with positron-emission/computed tomography simulation for stereotactic body radiation therapy. J Appl Clin Med Phys 2008;9:2763. [PubMed]
- Murphy MJ, Balter J, Balter S, et al. The management of imaging dose during image-guided radiotherapy: report of the AAPM Task Group 75. Med Phys 2007;34:4041-63. [PubMed]
- Wang JZ, Rice R, Pawlicki T, et al. Evaluation of patient setup uncertainty of optical guided frameless system for intracranial stereotactic radiosurgery. J Appl Clin Med Phys 2010;11:3181. [PubMed]
- Cerviño LI, Detorie N, Taylor M, et al. Initial clinical experience with a frameless and maskless stereotactic radiosurgery treatment. Pract Radiat Oncol 2012;2:54-62. [PubMed]
- Cerviño LI, Pawlicki T, Lawson JD, et al. Frame-less and mask-less cranial stereotactic radiosurgery: a feasibility study. Phys Med Biol 2010;55:1863-73. [PubMed]
- Peng JL, Kahler D, Li JG, et al. Characterization of a real-time surface image-guided stereotactic positioning system. Med Phys 2010;37:5421-33. [PubMed]
- Noël G, Simon JM, Valery CA, et al. Radiosurgery for brain metastasis: impact of CTV on local control. Radiother Oncol 2003;68:15-21. [PubMed]
- McDuff SG, Taich ZJ, Lawson JD, et al. Neurocognitive assessment following whole brain radiation therapy and radiosurgery for patients with cerebral metastases. J Neurol Neurosurg Psychiatry 2013;84:1384-91. [PubMed]
- Pan H, Cerviño LI, Pawlicki T, et al. Frameless, real-time, surface imaging-guided radiosurgery: clinical outcomes for brain metastases. Neurosurgery 2012;71:844-51. [PubMed]
- Bhatnagar AK, Flickinger JC, Kondziolka D, et al. Stereotactic radiosurgery for four or more intracranial metastases. Int J Radiat Oncol Biol Phys 2006;64:898-903. [PubMed]
- Schomas DA, Roeske JC, MacDonald RL, et al. Predictors of tumor control in patients treated with linac-based stereotactic radiosurgery for metastatic disease to the brain. Am J Clin Oncol 2005;28:180-7. [PubMed]
- Lawson JD, Fox T, Waller AF, et al. Multileaf collimator-based linear accelerator radiosurgery: five-year efficiency analysis. J Am Coll Radiol 2009;6:190-3. [PubMed]
- Nath SK, Lawson JD, Simpson DR, et al. Single-isocenter frameless intensity-modulated stereotactic radiosurgery for simultaneous treatment of multiple brain metastases: clinical experience. Int J Radiat Oncol Biol Phys 2010;78:91-7. [PubMed]


