A systematic review and meta-analysis of the efficacy and safety of long-acting release somatostatin analogs in patients with advanced neuroendocrine neoplasms
Introduction
Neuroendocrine neoplasms (NENs) are a heterogeneous group of cancers that originate from peptide neurons and neuroendocrine cells. Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) are the most common type of NENs, and represent 55% to 70% of all neuroendocrine neoplasms (1). Some NENs have no specific symptoms, whereas others produce peptides that cause characteristic hormonal syndromes, such as flushing and diarrhea. Regardless of the presence of functional or non-functional tumors, the only treatment that completely cures NEN is surgical resection. However, many patients have distant metastases and unsuitable for operation at the time of diagnosis (2). For this group of NEN patients, the best treatment is drug therapy, including palliative and antitumor treatment. Few medical treatments have been proven to be effective in patients with advanced NENs; however somatostatin analogs (SSAs) may be effective.
Somatostatin is an endogenous antiproliferative hormone that inhibits tumor cell division and induces cell cycle arrest and apoptosis (3). An SSA is a type of synthesized somatostatin that exhibits actions similar to that of somatostatin (4). Both octreotide long-acting release (LAR) and lanreotide LAR are commonly used in clinical practice to relieve NEN patient symptoms. In addition, the antiproliferative function of these drugs for advanced NENs has been studied (5). Multiple clinical trials have been performed to verify that SSAs delay tumor progression and prolong survival. However, these trials lacked a sufficient number of patients, with only dozens participating in each trial. Prior to this study, no meta-analyses had been performed to quantify the effectiveness of SSAs in advanced NENs. Therefore, a systematic review and meta-analysis was undertaken to evaluate the efficiency of SSAs in delaying tumor progression and prolonging survival time.
Methods
This meta-analysis will be performed (follows) the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Figure S1).
Study selection
A systematic search of all relevant literatures published until May 2016 was performed using 3 online databases: PubMed, Cochrane Library and Chinese BioMedical Literature (CBM). The key search terms were used in various combinations, and included “somatostatin LAR”, “octreotide LAR”, “lanreotide LAR”, “neuroendocrine tumors”, “lanreotide”, “somatostatin analogs”, and “neuroendocrine neoplasms”. The article type was limited to “clinical trial”. All searches and literatures selections were independently conducted by two investigators (Zheng and Wang).
Inclusion criteria
All studies of patients with advanced NENs administered SSAs LAR were included. Both retrospective and prospective studies were included. All included studies were assessed by two authors independently (Zheng and Wang).
Exclusion criteria
All studies that failed to fulfill the inclusion criteria were excluded. Other exclusion criteria included the following: (I) the presence of non-NEN tumors in any patient in the trial; (II) the lack of a pathological diagnosis in patients in the trial; (III) the lack of progression-free survival (PFS) or time to progression (TTP) measurements as end points; (IV) non-clinical trial article types; and (V) articles that were not written in English or Chinese.
Quality assessment
Because randomized controlled trials (RCTs) and non-RCTs were included in this meta-analysis, we used the Jadad scale to assess the quality of the RCTs, whereas the methodological index for non-randomized studies (MINORS) scale (6) was used to assess non-RCTs. If two independent evaluations conflicted, all authors participated in a discussion to resolve the controversy.
Data extraction
Data from the shortlisted articles were extracted independently by two authors (Zheng and Wang) and entered into a pre-designed form after achieving a consensus. The main data reported included baseline demographics, clinical characteristics and study outcomes. Baseline demographics and clinical characteristics include the study type, the number of patients treated with somatostatin LAR and placebo, study period, sex ratio, tumor locations, and tumor differentiation. The study outcomes included hazard ratios (HRs) and 95% confidence intervals (CIs) for PFS/ TTP, adverse events (AEs) and tumor objective response rate (ORR). According to the method reported by Zhang et al. (7), we introduced the placebo group from a well-matched RCT as the control arm by considering the sample size and baseline data if a single-arm study was included. When possible, we contacted the authors to obtain original data via e-mail. For trials for which original data were not available, we extracted the data from the published Kaplan-Meier curves and then used the spreadsheet designed by Tierney et al. (8) to calculate the HRs for PFS/TTP.
Statistical analysis
Review Manager (RevMan) v5.3 (Cochrane Library) was used to perform the meta-analysis of the comparative study (Figure S2). I2 and Cochran’s Q tests were used to determine statistical heterogeneity. Fixed-effect models were used in analyses if the P value was greater than 0.1 and the I2 was less than 50%; otherwise, random-effect models were used. P values less than 0.05 were considered significant.
PFS/TTP was the most common outcome in these time-to-event clinical trials, particularly for drugs evaluated as tumor therapies. The HR of PFS/TTP is a suitable index for incorporation into the analysis (8). Subgroup analyses of PFS/TTP were performed between various SSAs. In addition, the odds ratio (OR) was used for ORR and the risk ratio (RR) was used for AEs.
Results
Trials included
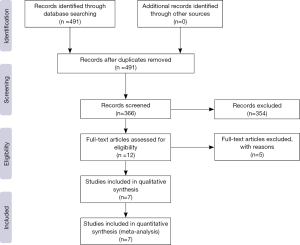
A total of 491 citations from three databases met our search strategies. Twelve articles were chosen based on a review of titles and abstracts. Reviews of full-text articles identified 7 that adequately matched the inclusion and exclusion criteria (Figure 1). These studies included two RCTs (9,10), one non-RCT (11) and four single-arm studies (12-15) with a total number of 788 patients who suffered from advanced GEP-NENs that were included in this meta-analysis. The quality assessment of the included articles and the characteristics of the included patients are presented in Tables 1,2,3. We used the control arm of PROMID (9) as the control arm for the 4 single-arm studies.
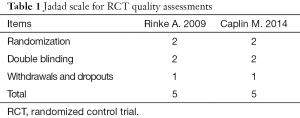
Full table
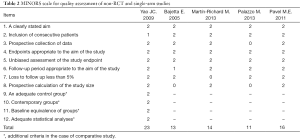
Full table
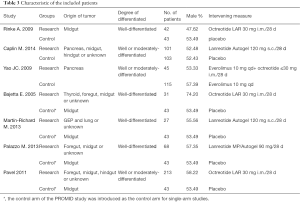
Full table
Effect of SSA versus placebo on PFS/TTP and ORR
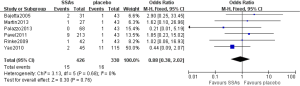
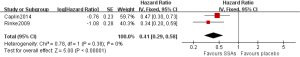
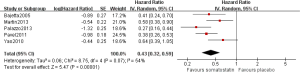
As shown in Figure 2, pooled HR analysis was performed among two RCTs, one non-RCT and four single-arm studies. A significant benefit of treatment with SSAs was noted with a pooled HR for PFS/TTP of 0.43 (95% CI: 0.36–0.51, P=0.14, I2=37%), indicating that SSAs can reduce the risk of neuroendocrine tumor progression by 57%. Moreover, the pooled HRs of the RCTs, non-RCT and single-arm studies were consistent with this value (Figures 3,4).
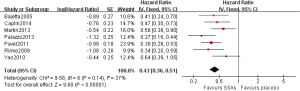
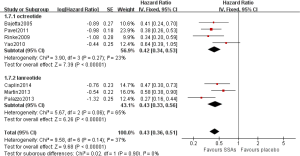
Subgroup analyses were performed to evaluate whether the pooled HRs of PFS/TTP differed for well differentiated and moderately differentiated. The pooled HRs of PFS/TTP were 0.42 (95% CI: 0.34–0.53, P=0.27, I2=23%) and 0.43 (95% CI: 0.33–0.56, P=0.06, I2=65%) for patients were well differentiated and moderately differentiated, respectively (Figure 5). Minimal differences in the pooled HRs of PFS/TTP between these two SSAs LAR were noted in the meta-analysis.
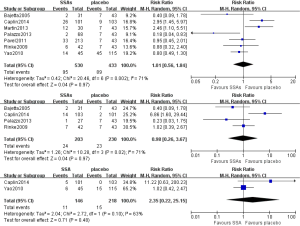
ORR, which includes complete response (CR) and partial response (PR), was used to evaluate the response of tumors according to either the Response Evaluation Criteria in Solid Tumor (RECIST) (16) or WHO criteria. Six articles reported ORR using radiological assessment. As shown in Figure 6, the pooled OR of the ORR was 0.88 (95% CI: 0.38–2.02, P=0.68, I2=0%), indicating that no statistically significant difference existed between tumor response to SSAs versus placebo.
Adverse effects
All articles reported AEs for the duration of SSA treatment. The most common AEs reported included diarrhea, nausea, abdominal pain, and hyperglycemia. Because single-arm studies were included, we pooled the RR of the available AEs of RCTs and single-arm studies with the introduced control arm. No statistically significant difference was noted for diarrhea, abdominal pain or hyperglycemia between the experimental and control groups (Figure 7).
Discussion
Although NENs are a rare group of malignant tumors, morbidity has rapidly increased over recent years. Most cases are diagnosed as advanced NENs and are unsuitable for operative treatment (2). Few medical treatments have proven to be effective for advanced NENs. Various cytotoxic drugs, such as cisplatin and etoposide, are effective in lung NEN (17); however, further research on the effectiveness of these treatments at other tumor sites is needed. SSAs exhibit antiproliferative activity similar to that of endogenous somatostatin (4). Although limited studies on SSAs for advanced NENs have been conducted, the efficacy and safety of these compounds for NENs have not been summarized. Therefore, we conducted this meta-analysis to evaluate the therapeutic potential of SSAs for NENs.
In vitro experiments that explored the antitumor effects of SSAs, in NENs demonstrated an antiproliferative effect for SSAs via inhibition of angiogenesis (18,19). Several clinical trials have been conducted to explore the validity of this effect in humans. Rinke et al. (9) demonstrated that SSAs delay tumor progression, thereby providing a foundation for the application of SSAs in clinical practice. However, these clinical trials lacked a sufficient number of patients to reach a comprehensive conclusion. In the present study, we combined all patients treated with SSAs in multiple clinical trials to verify the effectiveness of these compounds. A significant difference in PFS/TTP was noted between patients treated with and without SSAs. Although single-arm studies were included, little difference among the pooled HRs of PFS/TTP from all studies was noted; however, a difference between RCTs and studies without RCTs was noted. These data suggest that SSAs significantly delay tumor progression as reported.
Subgroup analyses were performed to detect the effect of SSAs on different differentiations in this meta-analysis. However, we observed similar pooled HRs, suggesting SSAs LAR is equally effective in well differentiated and moderately differentiated NENs. SSAs LAR possess intrinsic features that delay tumor progression and do not require a variety of differentiations.
The safety of SSAs is an important consideration when using these compounds to treat patients with NENs. Because SSAs can inhibit the release of specific hormones, they may cause endocrine metabolic disorders. AEs, such as diarrhea, nausea, abdominal pain and hyperglycemia, have observed during SSAs treatment (20,21), and these events were noted in the included studies. However, in our meta-analysis, we determined that SSA treatment did not increase the incidence of diarrhea, abdominal pain and hyperglycemia. Therefore, SSAs are safe for the treatment of NENs.
A limitation of this meta-analysis is the limited number of RCTs available. Thus, we included one non-RCT and four single-arm studies, which might reduce the degree of reliability. However, we attempted to contact the authors of each single-arm study to obtain the original follow-up data. Regrettably, some data were unavailable. Therefore, we extracted the data from published Kaplan-Meier curves, which may decrease the accuracy of the data.
In conclusion, this meta-analysis demonstrates that SSAs LAR are beneficial for patients with advanced NENs and significantly delayed tumor progression. Moreover, SSAs LAR are well tolerated and do not increase the incidence rate of AEs, such as diarrhea, abdominal pain and hyperglycemia. Additional RCTs are required to support this conclusion.
Acknowledgments
Funding: Wu Jieping Medical Foundation (No. 320.6750.13287); Science and Technology Planning Project of Guangdong Province (No.2014A020212591).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.09.43). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [Crossref] [PubMed]
- Kaltsas GA, Besser GM, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev 2004;25:458-511. [Crossref] [PubMed]
- Bousquet C, Lasfargues C, Chalabi M, et al. Clinical review: Current scientific rationale for the use of somatostatin analogs and mTOR inhibitors in neuroendocrine tumor therapy. J Clin Endocrinol Metab 2012;97:727-37. [Crossref] [PubMed]
- Theodoropoulou M, Zhang J, Laupheimer S, et al. Octreotide, a somatostatin analogue, mediates its antiproliferative action in pituitary tumor cells by altering phosphatidylinositol 3-kinase signaling and inducing Zac1 expression. Cancer Res 2006;66:1576-82. [Crossref] [PubMed]
- Susini C, Buscail L. Rationale for the use of somatostatin analogs as antitumor agents. Ann Oncol 2006;17:1733-42. [Crossref] [PubMed]
- Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003;73:712-6. [Crossref] [PubMed]
- Zhang X, Yang XR, Huang XW, et al. Sorafenib in treatment of patients with advanced hepatocellular carcinoma: a systematic review. Hepatobiliary Pancreat Dis Int 2012;11:458-66. [Crossref] [PubMed]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [Crossref] [PubMed]
- Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 2009;27:4656-63. [Crossref] [PubMed]
- Caplin ME, Pavel M, Ćwikła JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014;371:224-33. [Crossref] [PubMed]
- Yao JC, Lombard-Bohas C, Baudin E, et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol 2010;28:69-76. [Crossref] [PubMed]
- Bajetta E, Catena L, Procopio G, et al. Is the new WHO classification of neuroendocrine tumours useful for selecting an appropriate treatment? Ann Oncol 2005;16:1374-80. [Crossref] [PubMed]
- Martín-Richard M, Massutí B, Pineda E, et al. Antiproliferative effects of lanreotide autogel in patients with progressive, well-differentiated neuroendocrine tumours: a Spanish, multicentre, open-label, single arm phase II study. BMC Cancer 2013;13:427. [Crossref] [PubMed]
- Palazzo M, Lombard-Bohas C, Cadiot G, et al. Ki67 proliferation index, hepatic tumor load, and pretreatment tumor growth predict the antitumoral efficacy of lanreotide in patients with malignant digestive neuroendocrine tumors. Eur J Gastroenterol Hepatol 2013;25:232-8. [Crossref] [PubMed]
- Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet 2011;378:2005-12. [Crossref] [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [Crossref] [PubMed]
- Hanna N, Bunn PA Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol 2006;24:2038-43. [Crossref] [PubMed]
- Oberg KE, Reubi JC, Kwekkeboom DJ, et al. Role of somatostatins in gastroenteropancreatic neuroendocrine tumor development and therapy. Gastroenterology 2010;139:742-53, 753.e1.
- Walter T, Hommell-Fontaine J, Gouysse G, et al. Effects of somatostatin and octreotide on the interactions between neoplastic gastroenteropancreatic endocrine cells and endothelial cells: a comparison between in vitro and in vivo properties. Neuroendocrinology 2011;94:200-8. [Crossref] [PubMed]
- Figg WD, Thibault A, Cooper MR, et al. A phase I study of the somatostatin analogue somatuline in patients with metastatic hormone-refractory prostate cancer. Cancer 1995;75:2159-64. [Crossref] [PubMed]
- Breitschaft A, Hu K, Hermosillo Reséndiz K, et al. Management of hyperglycemia associated with pasireotide (SOM230): healthy volunteer study. Diabetes Res Clin Pract 2014;103:458-65. [Crossref] [PubMed]