
Is it useful to combine taxanes with targeted agents in mCRPC patients—have we hit the target in this phase II study with docetaxel and curcumin allowing going for a phase III study?
Ten percent to 20% of men diagnosed with prostate cancer will eventually develop castration-resistant prostate cancer (CRPC). Standard treatment for symptomatic metastatic CRPC (mCRPC) has consisted of chemotherapy-based regimens in combination with steroids for the past two decades. Docetaxel with daily prednisone is the recommended first-line therapy for symptomatic mCRPC (1). Docetaxel is the first treatment to have shown an increase in median overall survival (OS) for mCRPC. With a gain of 2–3 months OS (2-4), three key phase II randomized or III studies demonstrated the clinical benefit of docetaxel over the previous standard treatment, the mitoxantrone, an anthracedione (1) (Table 1).

Full table
However, only half of the patients will have an objective response with a median OS of 2 years before the emergence of new hormonotherapy treatments. Recently, highly effective novel therapies have been approved. Recent clinical trials focus on treatment before or after docetaxel. Five new therapies demonstrated an improvement in OS (5), three of them pre-docetaxel administration: sipuleucel-T (6), enzalutamide (7), abiraterone acetate (8) and four treatments in the post-docetaxel setting: radium-223 dichloride (9), abiraterone acetate (10), enzalutamide (11) and cabazitaxel (12). Cabazitaxel is the only drug to have been compared to docetaxel in a phase III trial; the results of the FIRSTANA study showed similar OS with both taxanes but different toxicity profiles (13). Combinations of docetaxel with new targeted agents have been studied in order to increase its therapeutic effects but without success, mainly because phase I went directly to phase III without selecting a specific population to increase the efficacy of the combination therapy of docetaxel plus targeted agent (Table 2).
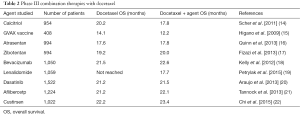
Full table
Mahammedi et al. reported the results of a phase II pilot study assessing the combination docetaxel, prednisone and curcumin (23). Pre-clinical studies suggested that curcumin increased docetaxel/prednisone cytotoxicity through downregulation of various cell cycle regulatory proteins (24), and seemed to provoke cell cycle growth arrest at the G1/S phase, by downregulation of cyclins (25). Sun et al. also suggested that the association of curcumin and docetaxel had pharmacokinetics propriety and increased taxanes therapeutic efficacy via the inhibitory role of curcumin on hepatic organic anion transporting polypeptide (OATP) 1B1, OATP1B3 and microsome activities (26). Thirty patients with mCRPC received docetaxel 75 mg/m2 every 3 weeks with dexamethasone premedication, prednisone 5 mg BID and 6,000 mg of curcumin daily for 7 consecutive days (from day −4 to day +2 of each cycle), as determined in the preliminary phase I study (27). An objective PSA response was observed in 59% (n=17) of patients. The objective response rate (ORR) was 40% (n=6) among the 15 patients with evaluable lesions. Those results were similar to those observed in the three main studies on docetaxel (2-4). There is no evidence to believe that this combination is more effective than docetaxel-prednisone alone in symptomatic mCRPC patients. Thus, those results are insufficient to recommend a phase III study.
An ancillary study assessed chromogranin A (CgA) and neuron-specific enolase (NSE) seric values, attempted to define prognostic markers to treatment response. CgA and NSE are frequently used as markers of neuro-endocrine (NE) differentiation. NE differentiation is correlated with a more aggressive disease (28). However, the prognostic role of CgA and NSE is unclear and its use in therapeutic decisions has not yet been defined. Twelve out of 22 patients had elevated baseline CgA levels, 6 NSE and 4 both markers. Those markers were correlated with PSA rate before treatment: patients with lower levels of PSA had significantly higher levels of NSE and lower CgA values. ORR and PSA response for patients with elevated CgA and NSE were the same as patients with no NE markers. However, NE variation was different according to the markers: a higher decrease rate was observed for NSE. The treatment seemed to be more active on patients with NSE marker than CgA. CgA and PSA had parallel evolutions. Before considering any phase III study for the combination of docetaxel, prednisone and curcumin, prognostic biomarkers are needed to define an eventual subgroup of mCRPC patients who could benefit from it.
Optimization of the docetaxel-prednisone combination is needed. However, past experiences of negative phase III with combination therapies are numerous (29). Finding a unique combination therapy effective for all patients is probably not realistic. Rather than associating new molecules with docetaxel in order to improve its response rates research might focus on understanding the biological differences in patients with mCRPC, enlightening the mechanism of response to docetaxel and identifying a targeted population who would benefit from this treatment. As it has been done with sunitinib and clear cell renal cell metastatic carcinoma (30), a translational clinical study identifying subgroups of patients based on proteomic and genomic analyses would be beneficial for a more optimal treatment. To our knowledge, translational clinical studies have yet to be lead in docetaxel treatment based regimens for mCRPC.
While resistance to androgen therapies via the detection of androgen receptor (AR) splice variant 7 messenger RNA (ARV7) in circulating tumor cells (CTC) has been investigated, predictive biomarkers of response or resistance to docetaxel in mCRPC are lacking (31). Recently, researchers have been able to understand various mechanism of resistance to docetaxel (32), such as an activated AR, activated tyrosine kinase receptors (RTK), anti-apoptotic signaling, aberrant proangiogenesis, unfavorable microenvironment, increased drug efflux, mutation of the drug target or drug resistant cancerous cell populations as NE differentiated cells.
A single drug combination will therefore be insufficient to encounter the diversity of these mechanisms. Multiple targets for new therapies are now being tested (29). Selecting the proper population should be a major concern in clinical trials. As molecular biology is making progress, more and more mechanism of resistance are identifying. In this light, clusterin is a chaperone molecule whose inhibition increased cell-sensitivity to many cytotoxic agents, including taxanes, by delaying the end of the mitotic phase. However, the Clusterin inhibitor custirsen failed to improve OS when combined with docetaxel. Further molecular compensatory mechanisms have recently been identified: the upregulation of cell cycle involved kinase Wee1. Simultaneous inhibition of Clusterin and Wee1 may improve synergistic responses of combinatorial regimens using taxanes (33).
Moreover, recent data suggested partial cross-resistance between taxanes and AR targeting agents (abiraterone acetate and enzalutamide) (34). On the other hand, some patients might benefit from a re-challenge with docetaxel. These results raise the question of treatment sequencing with three effective therapies before docetaxel and four approved treatments after docetaxel administration, determining the best sequence is still an issue.
In order to improve mCRPC survival, we should concentrate on understanding the diversity of the biological mechanism involved in the tumour-cell responses to treatment and on optimizing the therapeutic sequences in order to personalize the standard of care to patients’ specific cancer biology.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Hong-Chao He MD, PhD (Department of Urology, Shanghai Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.10.78). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol 1996;14:1756-64. [PubMed]
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502-12. [Crossref] [PubMed]
- Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004;351:1513-20. [Crossref] [PubMed]
- Oudard S, Banu E, Beuzeboc P, et al. Multicenter randomized phase II study of two schedules of docetaxel, estramustine, and prednisone versus mitoxantrone plus prednisone in patients with metastatic hormone-refractory prostate cancer. J Clin Oncol 2005;23:3343-51. [Crossref] [PubMed]
- Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract 2011;65:1180-92. [Crossref] [PubMed]
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411-22. [Crossref] [PubMed]
- Loriot Y, Miller K, Sternberg CN, et al. Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naive patients with metastatic castration-resistant prostate cancer (PREVAIL): results from a randomised, phase 3 trial. Lancet Oncol 2015;16:509-21. [Crossref] [PubMed]
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013;368:138-48. [Crossref] [PubMed]
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369:213-23. [Crossref] [PubMed]
- Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012;13:983-92. [Crossref] [PubMed]
- Fizazi K, Scher HI, Miller K, et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol 2014;15:1147-56. [Crossref] [PubMed]
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010;376:1147-54. [Crossref] [PubMed]
- Sartor AO, Oudard S, Sengelov L, et al. Cabazitaxel vs docetaxel in chemotherapy-naive (CN) patients with metastatic castration-resistant prostate cancer (mCRPC): A three-arm phase III study (FIRSTANA). J Clin Oncol 2016;34:abstr 5006.
- Scher HI, Jia X, Chi K, et al. Randomized, open-label phase III trial of docetaxel plus high-dose calcitriol versus docetaxel plus prednisone for patients with castration-resistant prostate cancer. J Clin Oncol 2011;29:2191-8. [Crossref] [PubMed]
- Higano C, Saad F, Somer B, et al. A phase III trial of GVAX immunotherapy for prostate cancer versus docetaxel plus prednisone in asymptomatic, castration-resistant prostate cancer (CRPC). J Clin Oncol 2009;27:LBA150
- Quinn DI, Tangen CM, Hussain M, et al. Docetaxel and atrasentan versus docetaxel and placebo for men with advanced castration-resistant prostate cancer (SWOG S0421): a randomised phase 3 trial. Lancet Oncol 2013;14:893-900. [Crossref] [PubMed]
- Fizazi K, Higano CS, Nelson JB, et al. Phase III, randomized, placebo-controlled study of docetaxel in combination with zibotentan in patients with metastatic castration-resistant prostate cancer. J Clin Oncol 2013;31:1740-7. [Crossref] [PubMed]
- Kelly WK, Halabi S, Carducci M, et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J Clin Oncol 2012;30:1534-40. [Crossref] [PubMed]
- Petrylak DP, Vogelzang NJ, Budnik N, et al. Docetaxel and prednisone with or without lenalidomide in chemotherapy-naive patients with metastatic castration-resistant prostate cancer (MAINSAIL): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 2015;16:417-25. [Crossref] [PubMed]
- Araujo JC, Trudel GC, Saad F, et al. Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY): a randomised, double-blind phase 3 trial. Lancet Oncol 2013;14:1307-16. [Crossref] [PubMed]
- Tannock IF, Fizazi K, Ivanov S, et al. Aflibercept versus placebo in combination with docetaxel and prednisone for treatment of men with metastatic castration-resistant prostate cancer (VENICE): a phase 3, double-blind randomised trial. Lancet Oncol 2013;14:760-8. [Crossref] [PubMed]
- Chi KN, Higano CS, Blumenstein BA, et al. Phase III SYNERGY trial: Docetaxel +/- custirsen and overall survival in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) and poor prognosis. J Clin Oncol 2015;33:abstr 5009.
- Mahammedi H, Planchat E, Pouget M, et al. The New Combination Docetaxel, Prednisone and Curcumin in Patients with Castration-Resistant Prostate Cancer: A Pilot Phase II Study. Oncology 2016;90:69-78. [Crossref] [PubMed]
- Cabrespine-Faugeras A, Bayet-Robert M, Bay JO, et al. Possible benefits of curcumin regimen in combination with taxane chemotherapy for hormone-refractory prostate cancer treatment. Nutr Cancer 2010;62:148-53. [Crossref] [PubMed]
- Sivanantham B, Sethuraman S, Krishnan UM. Combinatorial Effects of Curcumin with an Anti-Neoplastic Agent on Head and Neck Squamous Cell Carcinoma Through the Regulation of EGFR-ERK1/2 and Apoptotic Signaling Pathways. ACS Comb Sci 2016;18:22-35. [Crossref] [PubMed]
- Sun X, Li J, Guo C, et al. Pharmacokinetic effects of curcumin on docetaxel mediated by OATP1B1, OATP1B3 and CYP450s. Drug Metab Pharmacokinet 2016;31:269-75. [Crossref] [PubMed]
- Bayet-Robert M, Kwiatkowski F, Leheurteur M, et al. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol Ther 2010;9:8-14. [Crossref] [PubMed]
- Vashchenko N, Abrahamsson PA. Neuroendocrine differentiation in prostate cancer: implications for new treatment modalities. Eur Urol 2005;47:147-55. [Crossref] [PubMed]
- Oudard S. Progress in emerging therapies for advanced prostate cancer. Cancer Treat Rev 2013;39:275-89. [Crossref] [PubMed]
- Beuselinck B, Job S, Becht E, et al. Molecular subtypes of clear cell renal cell carcinoma are associated with sunitinib response in the metastatic setting. Clin Cancer Res 2015;21:1329-39. [Crossref] [PubMed]
- Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 2014;371:1028-38. [Crossref] [PubMed]
- Seruga B, Ocana A, Tannock IF. Drug resistance in metastatic castration-resistant prostate cancer. Nat Rev Clin Oncol 2011;8:12-23. [Crossref] [PubMed]
- Al Nakouzi N, Wang CK, Beraldi E, et al. Clusterin knockdown sensitizes prostate cancer cells to taxane by modulating mitosis. EMBO Mol Med 2016;8:761-78. [Crossref] [PubMed]
- van Soest RJ, van Royen ME, de Morrée ES, et al. Cross-resistance between taxanes and new hormonal agents abiraterone and enzalutamide may affect drug sequence choices in metastatic castration-resistant prostate cancer. Eur J Cancer 2013;49:3821-30. [Crossref] [PubMed]


