
Glioblastoma: does PET shed light to a difficult problem?
Glioblastoma is the most common primary brain tumor in the United States and despite aggressive multimodal therapy with maximum safe resection, radiotherapy in combination with concurrent and adjuvant temozolomide, the median survival of glioblastoma in clinical trial populations is 16 months. While the introduction of temozolomide into first-line standard of care (1) achieved some survival improvement, nearly all patients relapse and treatment options for recurrent disease remain limited and largely ineffective. Even under optimal circumstances with use of ‘state of the art’ diagnostic and therapeutic interventions, less than 15% of patients will survive 5 years (2-4).
Recently, there has been growing recognition for the need for novel, effective therapies for glioblastoma. Vice President Joseph Biden in a January 15, 2016 roundtable at the Abramson Cancer Center stated a goal of catalyzing greater investment, coordination, and collaboration in cancer therapy including a specific focus on advances in the treatment of glioblastoma. Currently validated treatments for glioblastoma and other central nervous system tumors are few in number and short on proven effectiveness. These treatments are also often toxic, threaten neurological function and hamper the quality of remaining life. The Brain Tumor Center at the MD Anderson has defined their Glioblastoma Moon Shot goal as an aim for better, safer therapeutics along with quadrupling the 5-year survival rate, from 10% to 40% over the next decade (5).
The ability to measure response to a treatment is a critical component in evaluating the efficacy of new therapies and identifying patients who require salvage therapy in a timely manner. Unfortunately, current diagnostic neuroimaging paradigms fail to reliably evaluate treatment response for glioblastoma. The initial landmark imaging evaluation guidelines—the Macdonald criteria—were established in 1990 and was based solely on the assessment of contrast-enhancement as a surrogate for tumor size. Contrast-enhancement is non-specific and simply reflects the degree of extravasation of a contrast agent across a disrupted blood brain: changes in contrast-enhancement may be attributable to true progression, imaging technique, treatment (surgery, radiation, or chemotherapy), steroids and parenchymal changes unrelated to the tumor (postsurgical changes, ischemia, seizures). Particularly with the use of multimodal therapy with radiation and temozolomide and new systemic therapies such as bevacizumab, new radiological phenomena including pseudoprogression and pseudoresponse have added further challenges to assessing treatment response.
In the context of clinical trials, accurate response assessment is essential. Misclassification of patients may lead to premature discontinuation of an actually effective agent, thereby withholding a potentially active treatment from the patient or inappropriate continuation of an inactive treatment that may have associated toxicities. Moreover, such misclassification may confound the data obtained in such studies and may lead to false conclusions with regards to the efficacy (or safety) of an investigated drug. An effort to address this challenge to accurately evaluate brain tumor response resulted in the formation of the Reponse Assessment in Neuro-Oncology (RANO) working group. In 2010, this group published updated guidelines for response assessment of high-grade gliomas incorporating additional MRI and clinical considerations, which addressed the recognized and accepted limitations of the Macdonald Criteria (6).
While these new criteria help standardize our approach for evaluating conventional MR images, the challenges of accurately assessing treatment response versus failure remain unaddressed. “Advanced” magnetic resonance based imaging techniques have the potential to provide anatomical, physiological, functional, metabolic and even genomic information that reflect treatment response evaluation and prognosis. Techniques including diffusion-weighted imaging (DWI), diffusion tensor imaging, perfusion MRI, and magnetic resonance spectroscopy (MRS) allow tumor assessment at the metabolic and physiologic level, but they have not yet been able to reliably differentiate tumor recurrence from radiation necrosis or pseudoprogression (7-12).
There has been general optimism for various advanced MR techniques to better characterize gliomas and differentiate tumor progression from pseudoprogression or radiation necrosis. For example, integration of the 2-hydroxyglutarate metabolite by MRS for the evaluation of IDH mutation status (13), T1ρ imaging (14), advanced diffusion imaging techniques including kurtosis (15), and “texture” based MR imaging analysis (16) have not yet been validated as being ready for routine clinical utilization.
Despite years of scientific work resulting in thousands of publications, there are very limited advanced MR features that have enough validated evidence to support clinical implementation to assist in evaluating tumor response. A major challenge in the field of MRI has been the ability to meaningfully compare findings across studies and institutions due to wide variability in image acquisition, post-processing, analysis and interpretation. Even for conventional MRI, a standardized recommended protocol has only recently been published in 2015 and implementation across clinical trials have only begun (17).
Functional molecular imaging with positron emission tomography (PET) has been in clinical use for the evaluation of brain tumors for over thirty years (18) providing complementary non-invasive metabolic imaging information about gliomas beyond current MR-based capabilities. Functional molecular imaging uses various tracers to visualize biological processes such as cell proliferation, membrane biosynthesis, glucose consumption, and uptake of amino acid analogs. Hence, PET provides additional insight beyond MRI into the biology and treatment response of gliomas and shows potential to be used as an adjunctive tool for noninvasive tumor grading, guiding surgical and radiotherapy treatment through improved tumor detection and delineation, evaluating treatment response, and prognostication.
Recently, both the Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology have collaborated towards a highly progressive and pragmatic step in the non-invasive evaluation of glioma patients by making formal recommendations on the use of PET in the management of glioma patients (19). The new guidelines define the recommended application of the most validated PET radiopharmaceuticals (18F-FDG, 11C-MET, 18F-FET, and 18F-FDOPA) in the assessment of tumor grading, delineation of glioma extent, evaluation for treatment planning including biopsy and resection, and assessment of treatment response including differentiation of progression from pseudoprogression. A series of trials has suggested that PET imaging is superior to MR imaging for each of these diagnostic paradigms (Table 1, from Alberts paper).
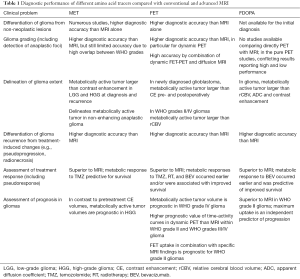
Full table
With the promise of PET as a key non-invasive imaging tool for glioma management and the support of the Neuro-Oncology community, it is time for the imaging community to collaboratively pursue rigorous multi-center clinical trials to generate stronger data that quantitatively demonstrates the clinical benefit of incorporating PET into glioma management. Recognizing the limitations and shortfalls of advanced MR imaging studies to-date, efforts now being pursued within the MR imaging community should be integrated into PET studies. This would entail a coordinated effort to implement standardized PET imaging protocols and to establish quality metrics that will enhance our ability to generate reproducible findings which would in turn support the need for PET utilization in clinical care in order to improve the outcomes of patients with glioblastoma.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Chao Wang (Department of Neurosurgery, Affiliated Hospital of Qingdao University, Qingdao, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.10.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96. [Crossref] [PubMed]
- Ostrom QT, Gittleman H, de Blank PM, et al. American Brain Tumor Association Adolescent and Young Adult Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012. Neuro Oncol 2016;18:i1-i50. [Crossref] [PubMed]
- Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol 2014;16:iv1-63. [Crossref] [PubMed]
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459-66. [Crossref] [PubMed]
- Glioblastoma|MOON SHOTS PROGRAM. Available online: http://www.cancermoonshots.org/cancer-types/glioblastoma/
- van den Bent MJ, Vogelbaum MA, Wen PY, et al. End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald's Criteria. J Clin Oncol 2009;27:2905-8. [Crossref] [PubMed]
- Bobek-Billewicz B, Stasik-Pres G, Majchrzak H, et al. Differentiation between brain tumor recurrence and radiation injury using perfusion, diffusion-weighted imaging and MR spectroscopy. Folia Neuropathol 2010;48:81-92. [PubMed]
- Hamstra DA, Galbán CJ, Meyer CR, et al. Functional diffusion map as an early imaging biomarker for high-grade glioma: correlation with conventional radiologic response and overall survival. J Clin Oncol 2008;26:3387-94. [Crossref] [PubMed]
- Hu LS, Baxter LC, Smith KA, et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol 2009;30:552-8. [Crossref] [PubMed]
- Hygino da Cruz LC Jr, Rodriguez I, Domingues RC, et al. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol 2011;32:1978-85. [Crossref] [PubMed]
- Lee HY, Na DG, Song IC, et al. Diffusion-tensor imaging for glioma grading at 3-T magnetic resonance imaging: analysis of fractional anisotropy and mean diffusivity. J Comput Assist Tomogr 2008;32:298-303. [Crossref] [PubMed]
- Vöglein J, Tüttenberg J, Weimer M, et al. Treatment monitoring in gliomas: comparison of dynamic susceptibility-weighted contrast-enhanced and spectroscopic MRI techniques for identifying treatment failure. Invest Radiol 2011;46:390-400. [Crossref] [PubMed]
- Choi C, Ganji SK, DeBerardinis RJ, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med 2012;18:624-9. [Crossref] [PubMed]
- Kettunen MI, Sierra A, Närväinen MJ, et al. Low spin-lock field T1 relaxation in the rotating frame as a sensitive MR imaging marker for gene therapy treatment response in rat glioma. Radiology 2007;243:796-803. [Crossref] [PubMed]
- Jiang R, Jiang J, Zhao L, et al. Diffusion kurtosis imaging can efficiently assess the glioma grade and cellular proliferation. Oncotarget 2015;6:42380-93. [PubMed]
- Skogen K, Schulz A, Dormagen JB, et al. Diagnostic performance of texture analysis on MRI in grading cerebral gliomas. Eur J Radiol 2016;85:824-9. [Crossref] [PubMed]
- Ellingson BM, Bendszus M, Boxerman J, et al. Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro Oncol 2015;17:1188-98. [PubMed]
- Patronas NJ, Di Chiro G, Brooks RA, et al. Work in progress: [18F] fluorodeoxyglucose and positron emission tomography in the evaluation of radiation necrosis of the brain. Radiology 1982;144:885-9. [Crossref] [PubMed]
- Albert NL, Weller M, Suchorska B, et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol 2016;18:1199-208. [Crossref] [PubMed]


