
New insights into patient radiosensitivity in relation to the biology of the ATM protein
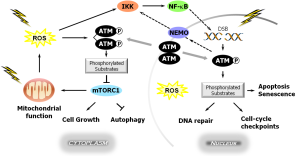
The cloning of the gene that encodes the mutated in ataxia-telangiectasia (ATM) protein in 1995 (1) led to a major overhaul of our scientific thinking in terms of the role of cell signaling pathways in the DNA damage response (DDR) network and in determining cellular and individual radiation sensitivity. ATM is best characterized as the apical kinase in the cellular response to ionizing radiation-induced DNA double strand breaks (DSBs), where it interacts with the Mre11-Rad50-Nbs1 (MRN) damage sensor complex and subsequently signals to a multitude of proteins involved in DNA repair, cell cycle checkpoint activation, apoptosis, and senescence-like growth arrest, by preferentially phosphorylating them on serine or threonine residues (2); Figure 1. Several excellent reviews have been published on various aspects of ATM signaling and function [e.g., (6-9)] and should be consulted for details. The number of ATM substrates is likely in the hundreds, so ATM signaling is complex and exerts profound effects on cellular function. Some important findings in regard to ATM stress signaling from the current perspective include:
- The observation that, like many other proteins, the activation of ATM in response to DSBs involves various post-translational modifications, including the early step of autophosphorylation between ATM dimer partners at serine-1981 to generate activated/phosphorylated monomers (10) and acetylation (e.g., by the Tip60 histone acetyltransferase) which plays a key role in linking the DDR to chromatin remodelling, which is itself an important component of the DDR (11);
- The identification of many downstream targets of the ATM kinase activity, which include p53, Chk2, KAP1 and the H2AX histone, the serine-139 phosphorylated form of which (γH2AX) has become a widely-used biomarker for ionizing radiation-induced DSBs (6,8);
- The periodic wave-like nature of the recurring activation of ATM (and consequently of its substrates such as p53) by DNA damage, as well as its deactivation, e.g., by phosphatases such as WIP1 (12);
- The emerging realization that ATM’s location and function extends beyond its well-defined DDR roles in the nucleus (Figure 1). Indeed, ATM has been reported in the cytoplasm, especially of neuronal or neuron-like cells, and in sub-cellular organelles such as mitochondria, endocytotic vesicles, microsomes, peroxisomes and centrosomes (3,6,8) where it may exert a variety of DNA damage-independent activities. For example, cytoplasmic ATM has been implicated in the maintenance of cellular redox homeostasis, where it might function as a direct cytoplasmic sensor of reactive oxygen species (ROS), possibly involving cysteine oxidation (3), as well as participating in insulin signaling and in the modulation of synaptic function in neuronal cells (7). The mitochondrial ATM pool is involved in maintaining mitochondrial homeostasis and is activated by mitochondrial dysfunction (8). These extra-nuclear functions of ATM are presumed to contribute to some of the phenotypic features apparent in ATM (also known as Louis-Bar syndrome) patients (3);
- In addition to its pivotal role in DSB repair, ATM kinase activity may also play a role in the resolving other types of genotoxic stress (6).
These advances in our understanding of the fundamental biology of ATM will hopefully be translated into progress in preventing or controlling human disease. One such area of intense interest is in their application to guiding treatment decisions in the clinical practice of XRT for cancer. A “holy grail” here has long been to identify pre-treatment biomarkers that will predict an individual patient’s predisposition to adverse normal tissue toxicities [e.g., (13)]. Such biomarkers would not only potentially spare at-risk individuals from debilitating (and potentially life threatening) reactions but might also allow dose escalation to non-susceptible patients with the expectation of improved tumor control; indeed, a recent estimate suggests that the dose to the most resistant 40% of patients might be increased by ~20% (14).
The feasibility of cellular/molecular and subsequent genomic biomarker approaches to stratifying individual radiosensitivity was initially driven by the observation that at least some radiation susceptibility (“extreme”) is due to inherited genetic alterations associated with DNA damage processing proteins, such as with ATM itself (15). Not surprisingly, ATM, as a master regulator of the DDR, as well as other key DSB repair proteins, were among the most widely-studied candidate molecules in early biomarker studies, including radiogenomics approaches that largely focused on single nucleotide polymorphisms (SNPs) in such genes (13). Despite considerable efforts, biomarkers based on genes/proteins such as ATM proved insufficiently robust for clinical adoption (16). Although candidate-gene SNPs in some DDR genes, including ATM, were reported to be associated with excess normal tissue radiotoxicity in some instances, e.g., in some prostate and breast cancer patients (13), such studies were largely underpowered and lacked a validation cohort, and subsequent studies with larger cohorts failed to validate any of these DDR-gene SNPs as XRT toxicity biomarkers (17,18). A particularly interesting example relates to the ATM G>A Asp1853Asn (rs1801516) SNP which is located in a highly conserved element in the ATM gene. It results in a non-conservative amino acid substitution from an acidic aspartic acid to a polar asparagine in exon 39, such that it might have functional consequences, and it was therefore a particular focus of candidate gene studies. Although the ATM rs1801516 variant was associated with normal tissue toxicity after XRT in some small studies, two meta-analyses looking at this SNP in larger cohorts were contradictory [reviewed in (19)].
So far, 2016 has proven to be a notable year for ATM in the translational XRT domain with the publication of two very provocative papers. The first of these (19), from members of the International Radiogenomics Consortium (RgC), was made possible by the compilation of a very large and well-annotated multicenter patient cohort. This resource has facilitated not only agnostic genome-wide association studies that use high-throughput microarrays incorporating large numbers of validated reference SNPs [e.g., (17)] but has also enabled well-powered studies of individual candidate-gene SNPs. Indeed, such a meta-analysis of the ATM rs1801516 SNP was undertaken by RgC investigators who examined DNA samples obtained from 5,456 patients: 2,759 breast cancer XRT patients from 11 cohorts; and 2,697 prostate cancer XRT patients from 6 cohorts; in relation to ~200,000 individual toxicity gradings spanning 8 acute/late endpoints (19). The study showed a significant association between the ATM rs1801516 SNP (Asn variant allele carriers) and increased risk of overall toxicity, acute toxicity, late toxicity, acute skin toxicity, acute rectal toxicity, telangiectasia, and fibrosis, but not late rectal toxicity. Associations were stronger for acute than late endpoints.
Another remarkable series of observations in regards to translating advances in our knowledge of the biology of ATM to the prediction of inter-individual variability in normal-tissue complications following XRT was also published in 2016, this time by Granzotto and colleagues from the COPERNIC project (20). The study looked at 117 non-transformed fibroblast strains derived from skin biopsy specimens obtained largely from patients receiving XRT for various cancers, but included cells from some cancer-free (control) and radiation-hypersensitive individuals with known rare predisposing genetic DDR defects. Three cellular end points were examined up to 24 h after in-vitro exposure of fibroblasts to 2 Gy of γ rays: micronuclei levels; phospho-ATM (serine-1981) foci, which are especially informative for the early recognition of DSBs; and γH2AX foci, which are also informative for the later stages of DSB repair. These molecular readouts were compared to the severity of clinical complications graded using standard scales and were considered in the context of three general “groups” of patient response: (I) radioresistant (grade 0 and controls); (II) moderately radiosensitive/“reactors” (grades 1, 2, 3 and 4); and (III) hyper-radiosensitive, which includes fatalities (grade 5) and individuals with known genetic radiosensitivity syndromes. The main findings can be summarized as follows:
- Cellular micronuclei levels at 24 h after a 2-Gy exposure could discriminate among patients in groups I, II, and III but not inter-individual differences in the grade of response within group II;
- Residual (24 h) γH2AX foci performed similarly to micronuclei, albeit better;
- The peak level of phospho-ATM foci (pATMmax) within the window of 10–60 min was the best single-parameter predictor and could discriminate among the four severity grades within group II;
- The combination of residual (24 h) γH2AX and pATMmax provided an even better discrimination of patient radiosensitivity; it also allowed the clearest demarcation between the two sub-sets of hyper-radiosensitivity that comprise group III responses, namely ATM-like with defects in both DSB recognition and repair, and those related to severe DSB-repair defects such as seen in Ligase IV syndrome patients.
Importantly, these relationships were independent of tumor site and, as with the RgC ATM rs1801516 SNP study outlined above (19), were applicable to both acute and late complications. The latter scenario is not unanticipated given that ATM, by virtue of its apical role in the cell-autonomous DDR, will impact on all complications to which cell death contributes, but ATM is also an active participant in ionizing radiation-induced inflammatory/immune signaling, e.g., involving NF-κB (Figure 1), as well as in the TGF-β pathway that plays an important role in the development of late toxicities, notably fibrosis (5,6,21). The superiority of the combination approach integrating pATMmax and residual (24 h) γH2AX foci measurements for estimating individual patient risk of adverse effects (20) may well relate to its ability to inform for both DSB recognition and repair defects, either or both of which may contribute to the elevation of such risk in different patients.
The COPERNIC patient-derived fibroblast study (20) was motivated in part by a mechanistic study from members of that group which suggested that high levels of both ATM and phospho-ATM (serine-1981) are present in the cytoplasm of non-stressed fibroblasts, based on immunofluorescence data (22). These authors proposed an alternative model for the early steps of DDR activation following ionizing radiation exposure in which cytoplasmic ATM dimers undergo oxidation and monomerization associated with serine-1981 phosphorylation, and then rapidly translocate to the nucleus where they engage in DSB processing; these events were hypothesized to be a critical determinant of cellular radiosensitivity (22). The authors note that their hypothesis is contrary to the broadly-accepted notion that the ATM protein is largely located and activated in the nucleus in response to DNA damage, as described above. Indeed, several earlier studies in various human cell types (including primary human fibroblasts) involving biochemical fractionation or fluorescently-tagged proteins visualized by fluorescence microscopy suggested ATM to be located predominantly in the nucleus under non-stressed conditions [e.g., (23,24) and references therein], and this paradigm is widely held [e.g., (7,9)]. The reasons for this discrepancy remain to be identified, although it is not clear why such high levels of monomeric phospho-ATM should be present in the cytoplasm of non-irradiated cells, as is suggested in Figure 4B of the recent paper from the COPERNIC investigators (20).
Interestingly, but contrary to the conversion of ATM dimers to active monomers seen in response to DNA damage/DSBs (10), the activation of ATM in response to ROS in primary human fibroblasts in the absence of DSBs (γH2AX foci) was reported to be accompanied by serine-1981 phosphorylation but not monomerization; rather, activation by ROS involved the direct oxidation of a critical cysteine residue (cysteine-2991) in ATM and the formation of a homodimer covalently cross-linked by intermolecular disulfide bonds (4). The sub-cellular compartmentalization of this mechanism was not studied. The relationship (if any) between these pathways of ATM activation remains to be established, but it is important to recognize that cells exposed to ionizing radiation will experience (and thus be responding to) DNA damage and ROS accumulation in various sub-cellular compartments concurrently (Figure 1).
In addition to its ability to predict the risk of XRT-induced complications with high precision, the ideal assay for use in a hospital setting should be simple and fast. Advantages of the SNP approach include the use of only a DNA sample and the lack of requirement for cell irradiation or of specialized immunohistochemical or fluorescence imaging methodologies, with their attendant variability. A weakness relates to bridging the clinical accuracy gap from a parameter that required a huge patient cohort for validation of a modest effect size into the reality of an individual patient test on which decisions about clinical management can be made with some certainty. In the latter context, the cell-based phospho-ATM/γH2AX predictor seems to have a reasonably good ability to discriminate grades of response even within a relatively small cohort, and it thus may prove advantageous for the true “personalization” of patient management decisions. This may reflect the notion that phenotypic readouts such as γH2AX and/or phospho-ATM foci might be more informative by virtue of their integrating the activity of many critical steps in the DDR, any of which might be defective in a particular patient. A down-side is that this test does require sample culturing, in-vitro irradiation and monitoring over an extended period using relatively specialized techniques. In reality, a manageably-small panel of biomarkers, possibly including both cell- and DNA-based information, might prove the most suitable tool for clinical use, and the combination of both phenotypic (γH2AX/phospho-ATM) and genotypic (ATM rs1801516 SNP) parameters may prove more robust.
Finally, we should note that the COPERNIC study (20) was enriched for cases with higher grade toxicity, which might over-weight the importance of γH2AX/phospho-ATM as an assay approach. Conversely, the Andreassen SNP meta-analysis (19) provides no direct mechanistic insight into the association with higher toxicity; the presumed functional change in ATM must be explored in future studies.
In summary, the last two decades have seen enormous advances in our understanding of the biology of the ATM protein kinase which not only mediates the nuclear response to DSBs but is also involved in maintaining cellular homeostasis in the face of other types of stress, such as elevated ROS levels. ATM activation is clearly a complex process both in space and time, and has been the subject of a recent review by Paull (9). The highly dynamic nature of these events is further highlighted by the observation that the DNA-damage induced activation of the NF-κB pathway noted earlier involves nuclear to cytoplasmic shuttling of ATM, with activated NF-κB going in the other direction (5) (Figure 1), and by the periodicity of such events in individual cells (12). However, there are clearly some contradictions in our understanding of the mechanisms of ATM nuclear and cytoplasmic activation and signaling that still need to be resolved. Regardless of the whether the delayed/attenuated formation of phospho-ATM foci seen in many radiosensitive patients (20) relates to the hypothesized “nucleoshuttling” mechanism of ATM activation, as opposed to being a primarily nuclear phenomenon, the findings of Granzotto et al. (20) represent a major advancement in the ability to utilize cellular-phenotypic readouts to estimate a patient’s risk of experiencing adverse normal tissue toxicities. Indeed, it is remarkable that two very different facets of a single molecule—albeit a very important one—ATM—can emerge from among the huge number of potential determinants of radiosensitivity and demonstrate a correlation with a high level of significance for both acute and late effects. What, if any, might be the relationship between the observed defects in DSB recognition as visualized by early phospho-ATM foci dynamics and the non-conservative rs1801516 SNP in exon 39 of the ATM gene, e.g., with respect to its importance for interactions of ATM with its binding partners, would be very interesting to evaluate, as would the SNP-type of these reactor patients in the COPERNIC study.
Acknowledgments
We apologize to the original authors of work that we have necessarily cited through review articles for brevity.
Funding: This article was supported in part by funding from the Canadian Breast Cancer Foundation-Prairies/NWT region (RF 2011-18) and from Prostate Cancer Canada (grant D2013-36).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Hongcheng Zhu (Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.10.23). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Savitsky K, Bar-Shira A, Gilad S, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 1995;268:1749-53. [Crossref] [PubMed]
- Mirzayans R, Andrais B, Scott A, et al. Ionizing radiation-induced responses in human cells with differing TP53 status. Int J Mol Sci 2013;14:22409-35. [Crossref] [PubMed]
- Alexander A, Walker CL. Differential localization of ATM is correlated with activation of distinct downstream signaling pathways. Cell Cycle 2010;9:3685-6. [Crossref] [PubMed]
- Guo Z, Kozlov S, Lavin MF, et al. ATM activation by oxidative stress. Science 2010;330:517-21. [Crossref] [PubMed]
- McCool KW, Miyamoto S. DNA damage-dependent NF-κB activation: NEMO turns nuclear signaling inside out. Immunol Rev 2012;246:311-26. [Crossref] [PubMed]
- Shiloh Y, Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol 2013;14:197-210. [Crossref]
- Bhatti S, Kozlov S, Farooqi AA, et al. ATM protein kinase: the linchpin of cellular defenses to stress. Cell Mol Life Sci 2011;68:2977-3006. [Crossref] [PubMed]
- Guleria A, Chandna S. ATM kinase: Much more than a DNA damage responsive protein. DNA Repair (Amst) 2016;39:1-20. [Crossref] [PubMed]
- Paull TT. Mechanisms of ATM Activation. Annu Rev Biochem 2015;84:711-38. [Crossref] [PubMed]
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 2003;421:499-506. [Crossref] [PubMed]
- Sun Y, Jiang X, Chen S, et al. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc Natl Acad Sci U S A 2005;102:13182-7. [Crossref] [PubMed]
- Batchelor E, Mock CS, Bhan I, et al. Recurrent initiation: a mechanism for triggering p53 pulses in response to DNA damage. Mol Cell 2008;30:277-89. [Crossref] [PubMed]
- Parliament MB, Murray D. Single nucleotide polymorphisms of DNA repair genes as predictors of radioresponse. Semin Radiat Oncol 2010;20:232-40. [Crossref] [PubMed]
- Scaife JE, Barnett GC, Noble DJ, et al. Exploiting biological and physical determinants of radiotherapy toxicity to individualize treatment. Br J Radiol 2015;88:20150172 [Crossref] [PubMed]
- Gatti RA. The inherited basis of human radiosensitivity. Acta Oncol 2001;40:702-11. [Crossref] [PubMed]
- Murray D, Parliament M. The Relationship Between DNA-Repair Genes, Cellular Radiosensitivity, and the Response of Tumors and Normal Tissues to Radiotherapy. In: Panasci L, Aloyz R, Alaoui-Jamali M. editors. Advances in DNA Repair in Cancer Therapy. New York: Springer-Verlag, 2013:75-128.
- Kerns SL, Stock RG, Stone NN, et al. Genome-wide association study identifies a region on chromosome 11q14.3 associated with late rectal bleeding following radiation therapy for prostate cancer. Radiother Oncol 2013;107:372-6. [Crossref] [PubMed]
- Barnett GC, Coles CE, Elliott RM, et al. Independent validation of genes and polymorphisms reported to be associated with radiation toxicity: a prospective analysis study. Lancet Oncol 2012;13:65-77. [Crossref] [PubMed]
- Andreassen CN, Rosenstein BS, Kerns SL, et al. Individual patient data meta-analysis shows a significant association between the ATM rs1801516 SNP and toxicity after radiotherapy in 5456 breast and prostate cancer patients. Radiother Oncol 2016; [Epub ahead of print]. [Crossref] [PubMed]
- COPERNIC project investigators. Influence of nucleoshuttling of the ATM protein in the healthy tissues response to radiation therapy: toward a molecular classification of human radiosensitivity. Int J Radiat Oncol Biol Phys 2016;94:450-60. [Crossref] [PubMed]
- Wang M, Saha J, Hada M, et al. Novel Smad proteins localize to IR-induced double-strand breaks: interplay between TGFβ and ATM pathways. Nucleic Acids Res 2013;41:933-42. [Crossref] [PubMed]
- Bodgi L, Foray N. The nucleo-shuttling of the ATM protein as a basis for a novel theory of radiation response: resolution of the linear-quadratic model. Int J Radiat Biol 2016;92:117-31. [Crossref] [PubMed]
- Gately DP, Hittle JC, Chan GK, et al. Characterization of ATM expression, localization, and associated DNA-dependent protein kinase activity. Mol Biol Cell 1998;9:2361-74. [Crossref] [PubMed]
- So S, Davis AJ, Chen DJ. Autophosphorylation at serine 1981 stabilizes ATM at DNA damage sites. J Cell Biol 2009;187:977-90. [Crossref] [PubMed]


