
S-1: changing the facets of adjuvant chemotherapy in pancreatic cancer?
From an oncologists view the treatment progress in curatively resected patients for pancreatic cancer (PDAC) has been frustrating so far. Although, the implementations of new standard of care chemotherapies in metastatic PDAC such as FOLFIRINOX (1) or Gemcitabine/Nab-Paclictaxel were a great advancement (2), adjuvant treatment efforts remained static. The use of adjuvant therapy is recommended by several medical societies (3-5) and is based on numerous trials: the CONKO-001 (6), ESPAC-1 (7) and ESPAC-3 (8) trials defined to start gemcitabine or infusional 5-FU monotherapy as the standard of care within 12 weeks post-operative, only differing in their respective toxicity profiles. These regimens have been a breakthrough at the time. However, novel and more tailored treatment algorithms are warranted to eventually reach a high response rate in only a subset of PDAC patients as “one pill fits all” seems to be an updated goal in PDAC.
A new era of adjuvant treatment?
In that sense, Uesaka and colleagues published a remarkable dataset from an adjuvant phase III trial, the JASPAC-01 trial (9). The study compared S-1, an orally taken fluoropyrimidine, to the standard of care, gemcitabine. Previously, S-1 already confirmed non-inferiority to gemcitabine in metastatic pancreatic cancer in a phase III trial in a Japanese/Taiwanese cohort (10).
The published data is based on a national (Japan), open-label, multi-centre, randomized controlled phase III trial. The primary end-point was overall survival (OS) and the statistical power was set on non-inferiority of S-1.
Patient characteristics were well adjusted between both groups. The per protocol population included 377 patients, randomized 1:1 (190 gemcitabine group, 187 S-1 group). Patients with R1-resection were also included and accounted for 14% and 12% in the gemcitabine and S-1 group, respectively. These numbers are slightly lower as in the CONKO-001 and the ESPAC-3 trial (CONKO-001: 19% R1-resections gemcitabine group; ESPAC-3: 35% R1-resections both treatment groups). Of note, the definition for R1 remains slightly different across these studies. Gemcitabine was used in the recommended standard dose (1,000 mg/m2 i.v. on d 1, 8, 15 every 4 weeks for six cycles). S-1 was administered according to the body surface area (BSA) (40, 50, 60 mg orally, b.i.d 28 days, followed by 14 days rest every 6 weeks for four cycles).
Using such treatment algorithm, the JASPAC-01 trial defines so far unreached numbers for relapse free survival (RFS) and OS (Table 1). The median OS was 25.5 months for gemcitabine and 46.5 months for S-1 treated patients (HR of 0.57 for mortality in favour of S-1). The estimated 5-year survival rate was 24.2% in the gemcitabine group and 43.6% in the S-1 group with a median RFS time of 11.3 months for gemcitabine and 22.9 months for S-1. Of note, the median OS times for gemcitabine in the JASPAC-01 trial was in the published range from 22.8 to 26.5 months (Table 1). Besides the nearly doubled survival time, subgroup analysis revealed a favourable outcome for patients with advanced T-stages (T3) and positive lymph nodes (N1) in the S-1 arm.
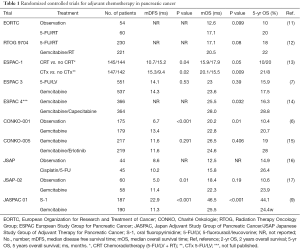
Full table
What about side effects? Indeed, both treatment regimen were comparable in most grade 3 and 4 adverse event besides more haematotoxicity in the gemcitabine group (Gem: neutropenia grade 3, 45% and grade 4, 27% of patients; S-1: neutropenia grade 3, 11% and grade 4, 2% of patients). Also, grade 3 and 4 infections were increased with gemcitabine. Anyhow, febrile neutropenia remained similar in both study arms as fatigue, anorexia, nausea and vomiting did. Not surprisingly S-1 was associated with increased gastrointestinal adverse events, mainly diarrhoea (grade 3, 4% and grade 4, 1% of the patients) but also stomatitis. Not named are side effects like rash or palmar-plantar erythrodysesthesia that have been previously described for S-1. Furthermore, tolerability was underpinned via a subgroup analysis for patients aged ≥65 years and by progressing quality of life improvement over time. In the gemcitabine group only 35% of the patients completed the planned treatment without dose reduction, compared to 59% in the S-1 group. Thus, S-1 outperforms gemcitabine not only in terms of efficiency but also tolerability at least in an Asian patient cohort.
In turn, one would expect that the JASPAC-01 trial fundamentally changes the field of adjuvant treatment to define S-1 as the new standard of care worldwide. However, a big question mark flanks “worldwide” as S-1 can only be announced for Asians as a standard and here in particular for Japanese. Thus, a potential weakness of the trial is, albeit the good design with a heterogeneous multi-centre distribution, the single nation realisation. The conclusions cannot be globally transferred, moreover even within Asia the transferability may be limited due to lacking data. But what may be the reasons for population limited effects? The key is probably the metabolism of S-1 by the cytochrome machinery having different genotypes at the CYP2A6 locus in Caucasians and East Asians. This in turn leads to higher toxicity due a faster conversion of tegafur to 5-FU, resulting in an increased area under curve for 5-FU, in the latter groups compared to the East Asian population (18-20). Furthermore, the tolerability of fluoropyrimidines seems to be generally reduced in Caucasians compared to Asians, maybe also due to diet (21). Such side effects limit dose escalation in Caucasians. In a small phase II trial with 27 metastatic PDAC patients S-1 (reduced to 30 mg/m2!, b.i.d. for 2 weeks, repeated every 3 weeks) showed an acceptable tolerability and even though comparison is not suitable median progression free survival and median OS were similar to the phase III GEST study (10,22). The recommended dosage for Caucasians is reduced to a far below range as it has been used and was well tolerated in the JASPAC-01 trial. In fact dosage according to the BSA would require even higher doses than used in the JASPAC-01 trial in most Caucasians based on their physique. Another hurdle to be taken is the limited availability of S-1 outside the Asian market resulting in restricted use and missing medical approval in several countries. At least in Europe S-1 is approved for gastric cancer in combination with cisplatin and teaches lessons on tolerability and effectiveness in a broader set of Caucasian patients. Anyhow, maybe upcoming subgroup analysis of the JASPAC-1 trial complemented with genetic analysis and biomarkers screened to predict a favourable S-1 metabolism for dose escalation will allow the identification and stratification of patients that primarily profit from S-1 independent of their ethnical background. As far as these data are lacking, the use of adjuvant S-1 in Caucasians has to be with caution and limited to clinical trials.
Moreover new treatment strategies tailored for a more Caucasian population are upcoming, having the capacity to change the standard of care such as the ESPAC-4 trial, propagating the combination of gemcitabine and capecitabine (Table 1). Furthermore, several trials are investigating even more intense treatment strategies like adjuvant FOLFIRINOX or Gemcitabine/Nab-Paclitaxel in the APACT trial (23,24), most of these studies have an adjacent biomarker project to define permissive subgroups. This probably will change our standard of care in the near future, in particular should allow more personalized strategies. Also combination approaches with S-1 have to be taken in account and may improve the efficacy as recently shown for the combination with oral leucovorin in metastatic PDAC, with altered application intervals (25). If these data is transferable remains elusive as far as combinations like S-1/gemcitabine did not improve efficacy but increased toxicity (10).
In summary, the data presented in the JASPAC-1 trial is remarkable and indeed changes the facets of treatment at least in an East Asian population. Recent studies identified several genetic subtypes differing not only in their molecular profile and morphology but also in their response to various treatments in metastatic PDAC (26-29). In turn, a biomarker being as simple as the affiliation of a patient to a certain population such as being East Asian is much more desirable than a molecular profile. In addition, we can anew learn from the trial that gemcitabine may not be the right player in the adjuvant treatment, in particular as it is associated with relatively high toxicity (only 35% of the patients completed the planned dosage). Furthermore, we can learn that the formulation of a drug within the same pharmacological group is of high importance, even more when thereby the bioavailability is prolonged or tolerability is improved resulting in higher total doses. Thereby the efficacy of S-1 may be driven by a higher potency against micrometastasis as shown in gastric cancer (30,31). The treatability of a subset of PDAC patients has improved by this study and gives hope that the running trials in this field may give us similar results and improve the landscape for the treatment of patients that are literally cured. But again a “one pill fits all” scenario moves further away and calls for more tailored approaches.
Acknowledgments
Funding: A Kleger is supported by the Deutsche Forschungsgemeinschaft (DFG, K.L. 2544/1-1 and K.L. 2544/1-2), the German Cancer Aid (111879), the Fritz-Thyssen Foundation (2015-00363), the Forschungskern SyStaR to A.K., BIU (Böhringer Ingelheim Ulm to A.K.), and the Else-Kröner-Fresenius-Stiftung (2011_A200). L Perkhofer is supported by a research fellowship of the Else-Kröner-Fresenius-Stiftung.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Gang Wang (Department of Pancreatic and Biliary Surgery, The First Affiliated Hospital of Harbin Medical University, Harbin, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.10.64). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v56-68. [Crossref] [PubMed]
- Tempero MA, Arnoletti JP, Behrman SW, et al. Pancreatic Adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2012;10:703-13. [PubMed]
- Yamaguchi K, Okusaka T, Shimizu K, et al. EBM-based Clinical Guidelines for Pancreatic Cancer (2013) issued by the Japan Pancreas Society: a synopsis. Jpn J Clin Oncol 2014;44:883-8. [Crossref] [PubMed]
- Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473-81. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 2010;304:1073-81. [Crossref] [PubMed]
- Valle JW, Palmer D, Jackson R, et al. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J Clin Oncol 2014;32:504-12. [Crossref] [PubMed]
- Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016;388:248-57. [Crossref] [PubMed]
- Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 2013;31:1640-8. [Crossref] [PubMed]
- Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 1999;230:776-82; discussion 782-4. [Crossref] [PubMed]
- Regine WF, Winter KA, Abrams R, et al. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol 2011;18:1319-26. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [Crossref] [PubMed]
- Neoptolemos JP, Palmer D, Ghaneh P, et al. ESPAC-4: A multicenter, international, open-label randomized controlled phase III trial of adjuvant combination chemotherapy of gemcitabine (GEM) and capecitabine (CAP) versus monotherapy gemcitabine in patients with resected pancreatic ductal adenocarcinoma. J ClinOncol 2016;34:abstr LBA4006.
- Sinn M, Liersch T, Gellert K, et al. CONKO-005: Adjuvant therapy in R0 resected pancreatic cancer patients with gemcitabine plus erlotinib versus gemcitabine for 24 weeks—A prospective randomized phase III study. J ClinOncol 2015;33:abstr 4007.
- Kosuge T, Kiuchi T, Mukai K, et al. A multicenter randomized controlled trial to evaluate the effect of adjuvant cisplatin and 5-fluorouracil therapy after curative resection in cases of pancreatic cancer. Jpn J Clin Oncol 2006;36:159-65. [Crossref] [PubMed]
- Ueno H, Kosuge T, Matsuyama Y, et al. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer 2009;101:908-15. [Crossref] [PubMed]
- Chuah B, Goh BC, Lee SC, et al. Comparison of the pharmacokinetics and pharmacodynamics of S-1 between Caucasian and East Asian patients. Cancer Sci 2011;102:478-83. [Crossref] [PubMed]
- Park SR, Kong SY, Nam BH, et al. CYP2A6 and ERCC1 polymorphisms correlate with efficacy of S-1 plus cisplatin in metastatic gastric cancer patients. Br J Cancer 2011;104:1126-34. [Crossref] [PubMed]
- Nakajima M, Fukami T, Yamanaka H, et al. Comprehensive evaluation of variability in nicotine metabolism and CYP2A6 polymorphic alleles in four ethnic populations. Clin Pharmacol Ther 2006;80:282-97. [Crossref] [PubMed]
- Haller DG, Cassidy J, Clarke SJ, et al. Potential regional differences for the tolerability profiles of fluoropyrimidines. J Clin Oncol 2008;26:2118-23. [Crossref] [PubMed]
- Schultheis B, Strumberg D, Bergmann L, et al. Results of a phase II trial of S-1 as first-line treatment of metastatic pancreatic cancer (CESAR-study group). Invest New Drugs 2012;30:1184-92. [Crossref] [PubMed]
- NCT01964430. Nab-paclitaxel and Gemcitabine vs Gemcitabine alone as Adjuvant Therapy for Patients with resected Pancreatic Cancer (apact). ClinicalTrialsgov. 2013.
- NCT02172976. Randomized Multicenter Phase II/III Study with Adjuvant Gemcitabine versus Neoadjuvant/Adjuvant FOLFIRINOX for Resectable Pancreas Carcinoma. ClinicalTrialsgov. 2014.
- Ueno M, Okusaka T, Omuro Y, et al. A randomized phase II study of S-1 plus oral leucovorin versus S-1 monotherapy in patients with gemcitabine-refractory advanced pancreatic cancer. Ann Oncol 2016;27:502-8. [Crossref] [PubMed]
- Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016;531:47-52. [Crossref] [PubMed]
- Moffitt RA, Marayati R, Flate EL, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet 2015;47:1168-78. [Crossref] [PubMed]
- Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015;518:495-501. [Crossref] [PubMed]
- Russell R, Perkhofer L, Liebau S, et al. Loss of ATM accelerates pancreatic cancer formation and epithelial-mesenchymal transition. Nat Commun 2015;6:7677. [Crossref] [PubMed]
- Eguchi T, Kodera Y, Nakanishi H, et al. The effect of chemotherapy against micrometastases and isolated tumor cells in lymph nodes: an in vivo study. In Vivo 2008;22:707-12. [PubMed]
- Yokoyama H, Nakanishi H, Kodera Y, et al. Biological significance of isolated tumor cells and micrometastasis in lymph nodes evaluated using a green fluorescent protein-tagged human gastric cancer cell line. Clin Cancer Res 2006;12:361-8. [Crossref] [PubMed]


