
G-protein coupled receptor PAR1 is overexpressed in glioma progenitor cells
Commentary
It is widely accepted that progenitor stem cells are the origin and initiators of different types of cancer that exhibit self-renewal, multipotency, and aggressive properties (1,2). This concept represents a different approach compared with previous theories, and the idea that cancer may be primarily driven by a small population of stem cells has important implications. For example, shrinking a tumor without killing the cancer stem cells may not be sufficient, since the remaining tumor cells are capable of re-growing, often with modified properties and resistance to previously used therapies. Furthermore, for the purpose of personalized therapy it is important to identify molecular traits that are specific to cancer stem cells, in comparison with normal stem cells, and this is a timely and achievable task.
Brain gliomas originating from tumor-initiating progenitor cells exhibit the capabilities of regrowth with new tumor characteristics. However, these tumors are unique due to the presence of the blood brain barrier (BBB). Normal brain hemostasis is guaranteed by prevention of free diffusion of molecules into brain capillary endothelial cells forming the BBB (3). Astrocytes are intimately associated with these endothelial cells, and contribute to the formation of a tight junction (4). In addition, astrocytes provide support and guidance for neural growth and differentiation (5). Thus, both brain capillary endothelial cells and the astrocytes that they are intimately associated with are components of the BBB.
Gliomas comprise the vast majority of malignant brain tumors. Low-grade (LG) forms (WHO grade II) are less malignant, but these slow-growing and infiltrating tumors can become more aggressive, evolving into glioblastoma multiform (GBM) (WHO grade IV), the most frequent and lethal glial neoplasm. High-grade (HG) gliomas include a panel of brain pathologies that includes oligodendroglioma and anaplastic astrocytoma (WHO grade III), as well as GBM (WHO grade IV). Current treatment modalities are rather limited, only modestly improving the survival of these patients (6). This is mainly due to resistance of gliomas to both radiotherapy and chemotherapy, as well as the rapid invasion properties of the glioma cells in brain tissue. The BBB poses an additional challenge, making delivery of effective drug doses for therapies to tumor cells is quite challenging. These characteristics have led to research focused on identification of specific molecular traits of the glioma progenitor cells.
Mammalian protease-activated receptors (PARs) are a subgroup of G protein-coupled receptors (GPCRs) that form a subfamily of PARs (7-11). PAR1 and PAR2 play a central role in epithelial tumor growth in a variety of epithelial malignancies (12-15). Whereas PAR2 is not considered a thrombin receptor (unlike PAR1, PAR3 and PAR4), the PAR1-tethered ligand SFLLRN is capable of transactivating PAR2 (16,17). Increasing evidence supports the notion that PAR1 and PAR2 exist in a close proximity and act as a functional unit while forming heterodimers (16-18). Consistently, PAR2 plays a dominant role, conferring function in tumor development while forming PAR1-PAR2 heterodimers, as well as in atherosclerotic plaque formation (19). Auvergne et al. successfully isolated glial progenitor cells (GPCs) by applying a monoclonal antibody; A2B5+, and sorting these cells from human gliomas (20). mRNA profiling identified a gene signature (including F2R PAR1) that distinguishes A2B5+ tumor progenitor cells from A2B5+ progenitor cells isolated from the normal brain white matter. In a recent Oncogene publication (21), this group demonstrated that shRNA silencing or pharmacological inhibition of PAR1 effectively attenuated glioma tumor expansion, in vivo.
Our understanding of PAR1 and its signaling pathway has vastly expanded. As PAR1 and PAR2 have a central task in tumor etiology, signal-binding motifs within their C-tails have been identified as critical sites for epithelial malignancies, and have been linked specifically to breast cancer growth. This is manifested via the association of Akt/PKB pleckstrin-homology (PH)-domain as a key signaling event of PARs. Other PH-domain signal-proteins such as Etk/Bmx and Vav3 also associate with PAR1 and PAR2 through their PH-domains (22). These PH-domain binding sites may provide a powerful platform for future therapeutic medicaments in cancer. Furthermore, PH-binding motifs may be utilized to identify powerful targets in cancer-driver GPCRs. Actually, peptides are more amenable efficient crossing of the BBB since a wide range of non-natural modifications may be applied, and a plethora of functional groups for site specific conjugation to a designated peptide or protein may be introduced (23-25). In addition, they are less immunogenic. Thus peptide shuttles may provide an efficient delivery mode, and may also be as a potent drug substance, following suitable modifications to optimize lipid solubility and hydrophilicity.
Pepducins are cell-penetrating peptides that act as intracellular modulators of PARs (26). They are comprised a short peptide derived from a GPCR third intracellular loop (e.g., PARs) tethered to a hydrophobic moiety. This structure allows pepducin lipopeptides to anchor in the cell membrane lipid bilayer and target the PAR/G protein interface. These peptides are also available as antagonists of PAR1.
Much attention has also been given to the involvement of serine proteases in brain injuries and other pathologies. In addition to tissue plasminogen activator (tPA), which is significant due to its therapeutic use in ischemic stroke, accumulating data have indicated that the serine protease thrombin, a major protease in the coagulation cascade, has important effects in brain injury (27). Once blood enters into the brain after injuries such as primary intracerebral hemorrhage and brain trauma, thrombin is immediately generated by cleavage from the circulating prothrombin in the blood. However, many traumatic and ischemic brain injuries that are associated with BBB disturbance occur without hemorrhage. In fact, it appears that the brain itself is a source for prothrombin production. Prothrombin mRNA is expressed in the cells of the nervous system (28), and upregulated following brain-related injuries (27,29,30). Taken together with the fact that mRNA for factor X is also generated in the brain (31), this implies that thrombin can be formed within the brain tissue with no breach in the BBB (Figure 1).
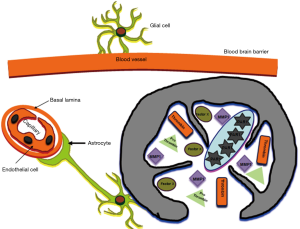
Other proteases that are centrally involved in the remodeling of tumor microenvironment stromal cells and with degradation of the extracellular matrix (ECM) are the zinc-dependent endopeptidases and matrix metalloproteinases (MMPs). Among these are MMP-1, as well as MMP-11 and MMP-19, which are highly upregulated in various malignancies, including gliomas (32). MMP-1 is produced by stromal cells rather than the tumor cells (33). Both thrombin and MMP1 are physiological activators of PAR1 (11,34).
Overall, advances in sequencing technology, gene array analysis, and bioinformatic tools, as well as the availability of sensitive and efficient means to isolate progenitor cancer stem cells, enable the identification of specific cancer progenitor molecular traits. These delicate means hold great promise in the fight against gliomas.
Acknowledgments
Funding: The authors acknowledge support by grants from the Israel Academy of Sciences and Humanities and Nofar Chief Israeli Scientist, Trade and Industrial Office to RB-S.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Ning Huang (Department of Neurosurgery, the Second Affiliated Hospital of Chongqing Medical University, Chongqing, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.11.14). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lamprecht S, Fich A. The cancer cells-of-origin in the gastrointestinal tract: progenitors revisited. Carcinogenesis 2015;36:811-6. [Crossref] [PubMed]
- Yang F, Xu J, Tang L, et al. Breast cancer stem cell: the roles and therapeutic implications. Cell Mol Life Sci 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Pardridge WM. Advances in cell biology of blood-brain barrier transport. Semin Cell Biol 1991;2:419-26. [PubMed]
- Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 1987;325:253-7. [Crossref] [PubMed]
- Vernadakis A. Neuron-glia interrelations. Int Rev Neurobiol 1988;30:149-224. [Crossref] [PubMed]
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96. [Crossref] [PubMed]
- Soh UJ, Dores MR, Chen B, et al. Signal transduction by protease-activated receptors. Br J Pharmacol 2010;160:191-203. [Crossref] [PubMed]
- Adams MN, Ramachandran R, Yau MK, et al. Structure, function and pathophysiology of protease activated receptors. Pharmacol Ther 2011;130:248-82. [Crossref] [PubMed]
- Nystedt S, Emilsson K, Wahlestedt C, et al. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci U S A 1994;91:9208-12. [Crossref] [PubMed]
- Rasmussen UB, Vouret-Craviari V, Jallat S, et al. cDNA cloning and expression of a hamster alpha-thrombin receptor coupled to Ca2+ mobilization. FEBS Lett 1991;288:123-8. [Crossref] [PubMed]
- Coughlin SR. Thrombin signalling and protease-activated receptors. Nature 2000;407:258-64. [Crossref] [PubMed]
- Bar-Shavit R, Turm H, Salah Z, et al. PAR1 plays a role in epithelial malignancies: transcriptional regulation and novel signaling pathway. IUBMB Life 2011;63:397-402. [Crossref] [PubMed]
- Booden MA, Eckert LB, Der CJ, et al. Persistent signaling by dysregulated thrombin receptor trafficking promotes breast carcinoma cell invasion. Mol Cell Biol 2004;24:1990-9. [Crossref] [PubMed]
- Even-Ram S, Uziely B, Cohen P, et al. Thrombin receptor overexpression in malignant and physiological invasion processes. Nat Med 1998;4:909-14. [Crossref] [PubMed]
- Versteeg HH, Schaffner F, Kerver M, et al. Protease-activated receptor (PAR) 2, but not PAR1, signaling promotes the development of mammary adenocarcinoma in polyoma middle T mice. Cancer Res 2008;68:7219-27. [Crossref] [PubMed]
- Blackhart BD, Emilsson K, Nguyen D, et al. Ligand cross-reactivity within the protease-activated receptor family. J Biol Chem 1996;271:16466-71. [Crossref] [PubMed]
- O'Brien PJ, Prevost N, Molino M, et al. Thrombin responses in human endothelial cells. Contributions from receptors other than PAR1 include the transactivation of PAR2 by thrombin-cleaved PAR1. J Biol Chem 2000;275:13502-9. [Crossref] [PubMed]
- Sevigny LM, Austin KM, Zhang P, et al. Protease-activated receptor-2 modulates protease-activated receptor-1-driven neointimal hyperplasia. Arterioscler Thromb Vasc Biol 2011;31:e100-6. [Crossref] [PubMed]
- Jaber M, Maoz M, Kancharla A, et al. Protease-activated-receptor-2 affects protease-activated-receptor-1-driven breast cancer. Cell Mol Life Sci 2014;71:2517-33. [Crossref] [PubMed]
- Auvergne RM, Sim FJ, Wang S, et al. Transcriptional differences between normal and glioma-derived glial progenitor cells identify a core set of dysregulated genes. Cell Rep 2013;3:2127-41. [Crossref] [PubMed]
- Auvergne R, Wu C, Connell A, et al. PAR1 inhibition suppresses the self-renewal and growth of A2B5-defined glioma progenitor cells and their derived gliomas in vivo. Oncogene 2016;35:3817-28. [Crossref] [PubMed]
- Kancharla A, Maoz M, Jaber M, et al. PH motifs in PAR1&2 endow breast cancer growth. Nat Commun 2015;6:8853. [Crossref] [PubMed]
- Oller-Salvia B, Sánchez-Navarro M, Giralt E, et al. Blood-brain barrier shuttle peptides: an emerging paradigm for brain delivery. Chem Soc Rev 2016;45:4690-707. [Crossref] [PubMed]
- Kastin AJ, Pan W, Maness LM, et al. Peptides crossing the blood-brain barrier: some unusual observations. Brain Res 1999;848:96-100. [Crossref] [PubMed]
- Bera S, Kar RK, Mondal S, et al. Structural Elucidation of the Cell-Penetrating Penetratin Peptide in Model Membranes at the Atomic Level: Probing Hydrophobic Interactions in the Blood-Brain Barrier. Biochemistry 2016;55:4982-96. [Crossref] [PubMed]
- Zhang P, Covic L, Kuliopulos A. Pepducins and Other Lipidated Peptides as Mechanistic Probes and Therapeutics. Methods Mol Biol 2015;1324:191-203. [Crossref] [PubMed]
- Xi G, Reiser G, Keep RF. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: deleterious or protective? J Neurochem 2003;84:3-9. [Crossref] [PubMed]
- Dihanich M, Kaser M, Reinhard E, et al. Prothrombin mRNA is expressed by cells of the nervous system. Neuron 1991;6:575-81. [Crossref] [PubMed]
- Riek-Burchardt M, Striggow F, Henrich-Noack P, et al. Increase of prothrombin-mRNA after global cerebral ischemia in rats, with constant expression of protease nexin-1 and protease-activated receptors. Neurosci Lett 2002;329:181-4. [Crossref] [PubMed]
- Citron BA, Smirnova IV, Arnold PM, et al. Upregulation of neurotoxic serine proteases, prothrombin, and protease-activated receptor 1 early after spinal cord injury. J Neurotrauma 2000;17:1191-203. [Crossref] [PubMed]
- Shikamoto Y, Morita T. Expression of factor X in both the rat brain and cells of the central nervous system. FEBS Lett 1999;463:387-9. [Crossref] [PubMed]
- Stojic J, Hagemann C, Haas S, et al. Expression of matrix metalloproteinases MMP-1, MMP-11 and MMP-19 is correlated with the WHO-grading of human malignant gliomas. Neurosci Res 2008;60:40-9. [Crossref] [PubMed]
- Conant K, St Hillaire C, Nagase H, et al. Matrix metalloproteinase 1 interacts with neuronal integrins and stimulates dephosphorylation of Akt. J Biol Chem 2004;279:8056-62. [Crossref] [PubMed]
- Boire A, Covic L, Agarwal A, et al. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell 2005;120:303-13. [Crossref] [PubMed]


