
INTEGRATE phase II trial: regorafenib vs. placebo in pretreated metastatic gastric cancer patients—is there anything new?
Gastric cancer ranks among the most frequent cancers and the third leading cause of cancer mortality in both sexes worldwide (1,2). Unfortunately in western Countries the majority of gastric tumours are diagnosed in an advanced stage, when outcome remains disappointing. In this setting chemotherapy still represents the standard of care with the notable exception of trastuzumab use in HER-2 over-expressing tumours (3-7). Recent progresses in the molecular characterization of gastric adenocarcinomas opened new insight for new treatment possibilities to be hopefully explored in the near future (8). Along with these potential treatment opportunities, targeting tumour angiogenesis still represents a therapeutic alternative deserving further clinical development despite the discouraging results from initial trials investigating the role of the anti-VEGF-A monoclonal antibody, bevacizumab (9-11). In fact more recently the use of ramucirumab (an anti-VEGFR-2 monoclonal antibody) demonstrated to improve the clinical outcome of metastatic gastric cancer patients failing first-line treatment, thus becoming one of the possible standard treatments in this setting (12-15).
Consistently with these findings, results from a Chinese phase III study suggested that apatinib, a VEGFR-2 tyrosine kinase inhibitor, might represent an effective second-line treatment thus reinforcing the hypothesis that tumour angiogenesis is a relevant therapeutic target for these patients (16). In this clinical scenario, Pavlakis and co-workers presented the results of the INTEGRATE trial a phase II randomised trial comparing regorafenib to placebo in metastatic gastric cancer patients refractory to one or two previous lines of treatment (17). The trial objective was to evaluate the clinical activity of regorafenib, an oral multikinase inhibitor that blocks the activity of several protein kinases, including kinases involved in tumour angiogenesis (VEGFR1, VEGFR2, VEGFR3, TIE2), oncogenesis (KIT, RET, RAF-1, BRAF) and in the tumour microenvironment (PDGFR, FGFR).
Primary endpoint was PFS, secondary endpoint included objective tumour response, clinical benefit status at two months, overall survival (OS), toxicity and quality of life. The study included a preplanned stratification for several covariates in order to define their role in the prediction of clinical outcome. Eligible patients were recruited from four Countries (Australia, New Zealand, Canada and South Korea). Ninety-seven patients were recruited in experimental arm, 50 patients in the control arm. More than half of the patients (58%) received either regorafenib or placebo as III line treatment, whereas the remaining patients received treatment as II line therapy. Most of the patients had ECOG PS 0 or 1. Nearly half of the patients (48%) in the experimental group had neutrophil-lymphocyte ratio (NLR) ≥3 (58% in the placebo group), 42% of the patients in the experimental group had VEGF-A >0.14 pg/mL (46% in the placebo group), 38% had an esophagogastric junction primary tumour. Median age was 63, 36% of the patients in the experimental group were South Korean (38% in the placebo group), 64% were from other Countries (62% in the placebo group). The trial reached its primary endpoint, patients in the regorafenib arm showed in fact an improved median PFS (2.6 vs. 0.9 months; HR 0.40; P<0.001). An OS trend in favour of the regorafenib arm was also reported, although this was not statistically significant (5.8 vs. 4.5 months; HR 0.74; P=0.147). No statistically significant differences were reported in both response rate and in the quality of life score. Findings from the study suggested that baseline NLR might have an independent prognostic role for OS and PFS (respectively HR 1.82, P=0.001 and HR 1.56, P=0.01), whereas basal VEGF-A plasmatic levels did not correlated with outcome. Although patients from South Korea performed globally better, the PFS advantage for regorafenib treatment was consistent across the different patients’ subgroups. The analysis of patients’ characteristics in the Integrate trial underscored, once again, clinical-pathological and disease-management differences between Asian and Caucasian gastric cancer patients. Compared to patients from Western Countries less South Korean patients had an esophagogastric junction primary tumour (4%), NLR and VEGF-A plasma concentrations were lower. On the contrary the numbers of previous lines for advanced disease were higher in the South Korean patients (83% vs. 43%).
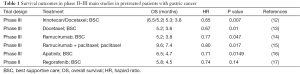
Although the Integrate trial had an undiscussed biological and clinical credibility in suggesting that regorafenib might have an interesting clinical activity in pre-treated advanced gastric cancer patients, several weaknesses in the trial design prevent us to draw definitive conclusions. The choice of a placebo standard arm in this setting is in fact particularly questionable. On the contrary according to current evidence second line treatment in refractory GC should be considered the standard approach in fit patients. Along with irinotecan and taxanes, ramucirumab either alone or in combination with paclitaxel represent the gold standard in good PS patients undergoing PD after first line treatment (Table 1).
Full table
These observations make study findings less interpretable and exclusively exploratory, especially when we consider the toxicity profile of regorafenib, which although manageable and apparently not affecting quality of life, should be carefully considered in the planning of a treatment strategy.
Unfortunately these questions about the use of regorafenib in the second-line treatment of gastric cancer patients are not likely to be answered in future trials. On the basis of the PFS advantage reported in the INTEGRATE phase II trial the authors designed the INTEGRATE II phase III trial (18). The Integrate II trial mostly differs from the phase II study in the primary endpoint (OS) and study population (adults with metastatic or locally recurrent gastro-oesophageal cancer failing or intolerant to two lines of prior anti-cancer therapy).
To date regorafenib cannot be considered a valid approach in the second line for gastric cancer patients; however findings from the Integrate trial represent a very strong basis for further trial. These results along with those deriving from trials analyzing the blockade of mechanisms related to the PD-1/PD-L1 and CTLA-4 pathways will help us designing a more efficient and more targeted treatment strategy for these patients (19-21).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Lei Huang (Department of Gastrointestinal Surgery, the First Affiliated Hospital of Anhui Medical University, Hefei, China; German Cancer Research Center (DKFZ), Heidelberg, Germany).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.11.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer, 2013. Available online: http://globocan.iarc.fr, accessed on 23/september/2016.
- Bray F, Ren JS, Masuyer E, et al. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer 2013;132:1133-45. [Crossref] [PubMed]
- Wagner AD, Unverzagt S, Grothe W, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2010;CD004064 [PubMed]
- Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-46. [Crossref] [PubMed]
- Guimbaud R, Louvet C, Ries P, et al. Prospective, randomized, multicenter, phase III study of fluorouracil, leucovorin, and irinotecan versus epirubicin, cisplatin, and capecitabine in advanced gastric adenocarcinoma: a French intergroup (Fédération Francophone de Cancérologie Digestive, Fédération Nationale des Centres de Lutte Contre le Cancer, and Groupe Coopérateur Multidisciplinaire en Oncologie) study. J Clin Oncol 2014;32:3520-6. [Crossref] [PubMed]
- Van Cutsem E, Boni C, Tabernero J, et al. Docetaxel plus oxaliplatin with or without fluorouracil or capecitabine in metastatic or locally recurrent gastric cancer: a randomized phase II study. Ann Oncol 2015;26:149-56. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Maeda K, Chung YS, Ogawa Y, et al. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer 1996;77:858-63. [Crossref] [PubMed]
- Scartozzi M, Giampieri R, Loretelli C, et al. Tumor angiogenesis genotyping and efficacy of first-line chemotherapy in metastatic gastric cancer patients. Pharmacogenomics 2013;14:1991-8. [Crossref] [PubMed]
- Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968-76. [Crossref] [PubMed]
- Kang JH, Lee SI, Lim DH, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 2012;30:1513-8. [Crossref] [PubMed]
- Ford HE, Marshall A, Bridgewater JA, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 2014;15:78-86. [Crossref] [PubMed]
- Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [Crossref] [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol 2016;34:1448-54. [Crossref] [PubMed]
- Pavlakis N, Sjoquist KM, Martin AJ, et al. Regorafenib for the Treatment of Advanced Gastric Cancer (INTEGRATE): A Multinational Placebo-Controlled Phase II Trial. J Clin Oncol 2016;34:2728-35. [Crossref] [PubMed]
- A Randomised Phase III Double-Blind Placebo-Controlled Study of Regorafenib in Refractory Advanced Gastro-Oesophageal Cancer (AGOC) (INTEGRATE II). Available online: https://clinicaltrials.gov/show/NCT02773524, accessed September, 27 2016.
- Puzzoni M, Silvestris N, Leone F, et al. The Immune Revolution in Gastrointestinal Tumours: Leading the Way or Just Following? Target Oncol 2016;11:593-603. [Crossref] [PubMed]
- Ralph C, Elkord E, Burt DJ, et al. Modulation of lymphocyte regulation for cancer therapy: a phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinoma. Clin Cancer Res 2010;16:1662-72. [Crossref] [PubMed]
- Moehler MH, Cho JY, Kim YH, et al. A randomized, open-label, two-arm phase II trial comparing the efficacy of sequential ipilimumab (ipi) versus best supportive care (BSC) following first-line (1L) chemotherapy in patients with unresectable, locally advanced/metastatic (A/M) gastric or gastro-esophageal junction (G/GEJ) cancer. J Clin Oncol 2016;34:abstr 4011.


