
Adding to the targeted therapy toolbox: BRAF and MEK inhibition in the treatment of BRAF V600E metastatic non-small cell lung cancer
Knowledge of the role of oncogenic driver mutations in tumor initiation and maintenance has transformed the treatment of non-small cell lung cancer (NSCLC). Given the availability of targeted therapies that are approved for first-line use, guidelines now recommend that all patients with non-squamous lung cancer undergo routine testing for mutations in the epidermal growth factor receptor (EGFR) gene and rearrangements in the anaplastic lymphoma kinase (ALK) gene (1-4). The success of targeted therapies for EGFR- and ALK-mutated NSCLC as well as the historically poor outcomes of patients with advanced disease has led to increased interest in identifying additional driver mutations in lung cancer that may similarly be targets for novel therapies. One such potential target is the BRAF oncogene, which encodes a serine-threonine protein kinase within the mitogen-activated protein kinase (MAPK) signaling pathway that regulates cell growth (5). Mutations in BRAF occur in 2–4% of NSCLC with predominance in adenocarcinoma (6-9). The clinical characteristics of patients with BRAF mutant NSCLC tend to be similar to those of patients with BRAF wildtype NSCLC. BRAF mutations occur in both males and females but favor older patients (age >60) and current or former smokers (8,10). At least half of BRAF mutations in NSCLC are characterized by the substitution of glutamic acid for valine at position 600 (V600E) within the BRAF protein, leading to constitutive activation of the kinase and subsequent tumorigenesis (7,9). Although the remaining non-V600E BRAF mutations are similarly thought to drive tumorigenesis in NSCLC, the efficacy of targeted therapies against these mutations is questionable, and clinical trials in other solid tumors have focused on patients with BRAF V600E mutations in particular (11-13).
Inhibitors of the V600E mutant BRAF kinase, including dabrafenib and vemurafenib, were initially approved for melanoma, which harbors BRAF mutations in >40% of cases (14,15). Based on the efficacy of BRAF inhibitors in this clinical setting and the success of other targeted therapies in NSCLC, there has been interest in pivoting towards the use of BRAF inhibitors for BRAF V600E mutant lung cancer. In Lancet Oncology, Planchard et al. recently published the two largest phase 2 studies to date evaluating the clinical activity and safety profile of BRAF monotherapy and combination BRAK-MEK inhibition, respectively, in previously treated NSCLC (16,17). A third cohort of patients receiving BRAF-MEK combination therapy in the first-line setting has yet to be reported. In the first of the two published studies, 78 patients with stage IV NSCLC who had progressed after one or more systemic therapies were enrolled from August 2011 to February 2014. Notable inclusion criteria included the presence of a BRAF V600E mutation as identified locally by Clinical Laboratory Improvement Amendments (CLIA) approved methods and an ECOG performance status 0-2. Patients with brain metastases that were <1 cm in size, untreated, and asymptomatic were allowed to enroll. All patients received dabrafenib 150 mg twice daily as monotherapy unless adverse events merited a dose reduction. By investigator assessment, the primary endpoint of overall response was achieved in 26 of 78 patients (33%; 95% CI: 23–45%, all partial responses). The majority of these responses (73%) were detectable by the time of the first patient assessment at 6 weeks from baseline. Disease control, defined as the number of patients achieving a response or stable disease for ≥12 weeks after the initiation of therapy, was reported in 45 patients (58%; 95% CI: 46–67%). Median progression-free survival (PFS) was 5.5 months, and median overall survival (OS) was 12.7 months.
In the second study, 59 patients with stage IV NSCLC who had progressed after one or more platinum-based systemic chemotherapy regimens were enrolled from December 2013 to January 2015. Inclusion and exclusion criteria were similar to the cohort described above. All patients were treated with dabrafenib 150 mg twice daily plus trametinib 2 mg daily unless dose reduction was warranted due to adverse events. Trametinib inhibits the mitogen-activated protein kinase kinase (MEK), a downstream effector of RAF within the MAPK pathway. An investigator-assessed overall response was documented in 36 of 57 eligible patients (63.2%; 95% CI: 49.3–75.6%), including two complete responses. Disease control was documented in 45 patients (78.9%; 95% CI: 66.1–88.6%), and PFS was 9.7 months. Although median duration of response was 9.0 months at the time of data cutoff, 18 of 36 responses were still ongoing, and the majority of these patients (approximately 16 of 18) had already been on therapy for at least 6 months. Survival data for this cohort is incomplete.
Prior to these results, studies of BRAF inhibition in NSCLC had been limited (Table 1). Early support for BRAF inhibition in NSCLC came from case reports of patients treated off-label with dabrafenib or vemurafenib (18-21). Subsequently, a retrospective analysis of the European BRAF cohort (EURAF) by Gautschi et al. reported outcomes in patients with BRAF-mutant NSCLC who had received BRAF monotherapy as first- or second-line treatment (13). Among 34 patients, 29 with V600E mutations, overall response rate (ORR) was 53% and disease control rate (DCR) was 85%. Although these results were striking, validation by prospective studies has been necessary. In a phase 1 study of dabrafenib monotherapy in various solid tumors, Falchook et al. accrued a single patient with NSCLC who achieved a partial response to dabrafenib with an 83% reduction in tumor size (22). In a larger phase 2 “basket trial” of vemurafenib in non-melanoma tumors, the ORR in a cohort of 20 patients with BRAF-mutant NSCLC (18 with V600E mutations) was 42%, and median PFS was 7.3 months (23). Data supporting MEK inhibition in NSCLC is even more limited by comparison. In a trial of patients with NSCLC, small cell lung cancer, and thymic malignancies treated with selumetinib monotherapy, the ORR was 11% in nine patients with NSCLC (24). However, the study included patients with mutations in any one of multiple RAS/RAF proteins including KRAS, HRAS, NRAS, or BRAF.

Full table
These few prospective trials have been limited by small patient numbers, which reflects the low incidence of BRAF mutations in NSCLC. Additionally, many of these studies were conducted as “basket trials” that included patients with multiple tumor types, which limits the inferences that can be drawn about the efficacy of therapy in lung cancer in particular. The studies conducted by Planchard et al. likely benefitted from multiple centers of enrollment as well as a more widespread understanding of the role of multiplex genotyping in improving patient outcomes in lung cancer (25). As a result, Planchard et al. were able to enroll relatively larger numbers of patients with BRAF-mutant NSCLC in each of their two cohorts reported thus far. With respect to the clinical characteristics of the patients enrolled, there was also fairly good correspondence with previous descriptions of individuals with BRAF V600E mutant NSCLC in the literature. In prior studies, median age at diagnosis has ranged from 63–67 years with BRAF mutations occurring predominantly in adenocarcinoma, which matches the cohorts enrolled in each of the studies from Planchard et al. (8-10). The percentage of never-smokers in each of the two cohorts (28% and 37%, respectively) was also similar to what has been reported previously (8,9,26).
With a sizeable cohort and fairly representative sample of patients enrolled, the results from Planchard et al. should set the current standard upon which the efficacy of BRAF monotherapy and BRAF-MEK combination therapy is judged. However, in considering whether dabrafenib or dabrafenib plus trametinib should be used routinely in the second-line treatment of BRAF-mutant NSCLC, it is important to understand what is known about the efficacy of currently approved second-line therapies since the studies from Planchard et al. were not randomized or controlled. When comparing results across trials, one must keep in mind that earlier studies of second-line therapy included patients with NSCLC regardless of tumor genotype whereas the studies from Planchard et al. were designed to evaluate only the subset of patients with NSCLC harboring BRAF V600E mutations. This caveat is especially important given that long-term survival of BRAF V600E mutant NSCLC has been described in select cases (27,28). In addition, at least one study has demonstrated a trend toward better outcomes among patients with NSCLC whose tumors harbor any BRAF mutation compared to those harboring other driver mutations or no mutations at all (10). On the other hand, in a nationwide French study of patients with NSCLC whose tumors were profiled for oncogenic mutations, outcomes among patients with BRAF mutant NSCLC receiving second-line therapy were poor (ORR 9%), with the majority receiving best supportive care only (26).
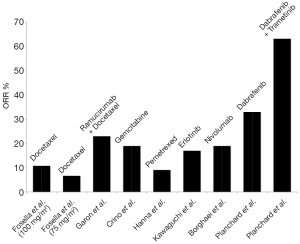
Per current guidelines, approved second-line therapies following disease progression include single-agent or combination chemotherapy (pemetrexed, docetaxel, gemcitabine, or ramucirumab plus docetaxel), targeted therapy (erlotinib) and newer immunotherapies (nivolumab, pembrolizumab) (1). Second-line chemotherapy agents in NSCLC have generally yielded poor results. Accounting for methodological differences, studies of single-agent gemcitabine reported ORRs ranging from 13–19% with median OS 26–34 weeks (29,30). Single-agent docetaxel by comparison was demonstrated in separate trials to be superior to best supportive care and single-agent vinorelbine or ifosfamide, respectively, but the highest ORR was only 10.8% and the longest median OS was 7.0 months in either of the two studies (31,32). Better outcomes were noted in a trial of docetaxel alone vs. docetaxel plus ramucirumab in which an ORR of 14% was reported for single-agent docetaxel (33). However, the authors of that study attributed such findings to the enrollment of patients with better performance status. Furthermore, the combination of ramucirumab and docetaxel was superior with respect to ORR (23% vs. 14%), DCR (64% vs. 53%), and median OS (10.5 vs. 9.1 months) compared to single-agent docetaxel. Single-agent pemetrexed has been comparable in regards to ORR (9.1% vs. 8.8%) and OS (8.3 vs. 7.9 months) compared to docetaxel (34).
As a second-line treatment option, erlotinib compared to placebo results in a greater ORR (8.9% vs. <0.1%) and median OS (6.7 vs. 4.7 months) (35). Compared to single-agent chemotherapy, however, the benefit of targeted therapy in this setting is less clear. A comparison of pemetrexed vs. erlotinib, for example, demonstrated similar outcomes with chemotherapy and targeted therapy (36). In the TAILOR study, ORR (15.5% vs. 3%) and DCR (44.3% vs. 22%) were higher in patients with wildtype EGFR NSCLC who were treated with docetaxel compared to erlotinib, and thus the benefit of targeted therapy in patients with wildtype tumors is questionable (37). With respect to newer anti-PD-1 immunotherapies, nivolumab compared to docetaxel has been associated with longer OS (12.2 vs. 9.4 months) and higher ORR (19% vs. 12%) (38). Herbst et al. reported similar benefits with pembrolizumab with median OS 10.4 months (2 mg/kg dose of pembrolizumab) and 12.7 months (10 mg/kg dose) and an ORR of 18% at both dosages (39). However, the study excluded patients with negative PD-1 expression <1% and found that the best outcomes were experienced by patients with PD-1 expression >50%.
In the context of these studies, dabrafenib monotherapy and dabrafenib plus trametinib both compare favorably to currently approved second-line therapies. The response rates reported for both dabrafenib alone and dabrafenib plus trametinib are higher than that which has been traditionally reported with either single-agent chemotherapy or erlotinib in EGFR wild-type patients in the second-line setting. Additionally, the median OS of 12.7 months in patients with BRAF mutant NSCLC receiving dabrafenib monotherapy is longer than the survival typically reported with second-line chemotherapy. While newer anti-PD-1 immunotherapies are promising, their efficacy is dependent on PD-1 expression in tumor cells, and it is unclear if they will represent a treatment option for all patients with BRAF-mutant NSCLC. Although some variability in results may be explained by differences in patient populations, enrollment sizes, and methods between studies, targeted therapy nonetheless seems to represent a significant treatment addition for the subset of patients with BRAF V600E mutant NSCLC. This furthermore highlights the importance of molecular testing in patients with NSCLC. To optimize the benefits of BRAF targeted therapy, clinicians must be able to accurately identify patients with NSCLC harboring targetable BRAF V600E mutations who would be candidates to receive dabrafenib or dabrafenib plus trametinib over other standard second-line therapy options for which responses are less robust (Figure 1).
For oncologists tasked with making treatment decisions for patients with BRAF V600E mutant NSCLC, the next dilemma is selecting between BRAF monotherapy vs. BRAF-MEK combination therapy. In melanoma, acquired resistance to BRAF monotherapy leads to eventual drug failure and disease progression (12). Preclinical studies in melanoma cell lines have demonstrated multiple mechanisms of acquired resistance including new mutations in NRAS or MEK and increased expression of COT, CRAF, or PDGF-α (40-45). The rationale for the combined use of BRAF and MEK inhibition is to delay acquired resistance by blocking two sites along the MAPK pathway, and studies in melanoma have demonstrated better outcomes with BRAF-MEK combination therapy compared to BRAF monotherapy (46). Planchard et al. caution against directly comparing the results of their two cohorts since each was studied independently. However, each study employed a similar methodological design and had a similar median duration of follow-up. The comparable baseline characteristics of each cohort with respect to age, sex, performance status, percentage of non-smokers, and histology also makes direct comparisons more palatable. It is worth noting that with respect to ethnicity, the two cohorts were not as well balanced with a greater percentage of patients of Asian ethnicity enrolled in the cohort receiving dabrafenib monotherapy (22% vs. 7%). The potential effect of this discrepancy on outcomes is not clear.
Across nearly all metrics, dabrafenib plus trametinib was superior with a higher ORR, higher DCR, and longer PFS than dabrafenib monotherapy. While the duration of response in each therapy group was similar (9.0 months for dabrafenib plus trametinib vs. 9.6 months for dabrafenib), 18 of the 36 patients receiving dabrafenib plus trametinib who achieved a response remained on therapy at the time of data cutoff. In addition, among all patients receiving dabrafenib plus trametinib, 17 out of 57 (30%) remained on therapy for >12 months. As pointed out by Planchard et al., the response rate of dabrafenib plus trametinib compared to that of dabrafenib monotherapy is closer to the response rates typically reported with other targeted therapies such as erlotinib and crizotinib, although some of these latter studies were conducted using targeted therapy as first-line treatment (2-4,47-50). With this in mind, combined dabrafenib plus trametinib should likely be the preferred option wherever possible but until a head-to-head trial of BRAF monotherapy and BRAF-MEK combination therapy is conducted in NSCLC, clinician experience, patient preference, and the safety profile of each therapy should always be considered. The poor outcomes of patients receiving second-line treatment for NSCLC in general should make even dabrafenib monotherapy an attractive option in cases where combination therapy is contraindicated.
The documented adverse events occurring in patients receiving dabrafenib monotherapy were similar to those reported in melanoma. Planchard et al. reported adverse events of grade 2 or worse in 45 of 84 (54%) patients. By comparison, in a phase 3 trial of dabrafenib monotherapy in melanoma patients, adverse events grade 2 or greater occurred in 53% of patients, the most common of which were skin-related, pyrexia, fatigue, headache, and arthralgia (12).The rate of grade 3 squamous cell carcinoma of the skin was less common in this study of patients with melanoma compared to the Planchard et al. cohort (12% vs. 4%). In the two studies from Planchard et al., patients receiving combination dabrafenib plus trametinib compared to those receiving dabrafenib monotherapy had higher rates of adverse events leading to drug discontinuation (12% vs. 6%), drug interruption (61% vs. 43%), and dose reduction (35% vs. 18%), which has been similarly reported in comparisons of BRAF monotherapy and BRAF-MEK combination therapy in melanoma (46). Serious adverse events were also more common in the cohort receiving combination therapy (56% vs. 42%). However, squamous cell carcinoma was much less common, occurring in only 4% of patients. Regardless of these differences, Planchard et al. reported that both therapies were tolerated well overall. With respect to serious adverse events, it is worth noting that one patient receiving dabrafenib monotherapy who was also on a factor Xa inhibitor died from an intracranial hemorrhage while one patient with a history of a cranial artery aneurysm receiving dabrafenib plus trametinib experienced a subarachnoid hemorrhage. Only the intracranial hemorrhage was attributed to the study drug. Although rare, three patients with cerebral hemorrhage were reported in a trial of dabrafenib plus trametinib in melanoma, and at least one case report of intracranial hemorrhage occurring in a patient receiving dabrafenib plus trametinib therapy has been described previously (46,51). While causality has not been established, the potential for such serious adverse events should be noted as use of dabrafenib and trametinib increases.
Conclusions
In conclusion, the recent studies from Planchard et al. shed new light onto the efficacy of targeted therapy as second-line treatment in patients with stage IV BRAF V600E mutant NSCLC. As existing second-line therapy options in NSCLC have traditionally been associated with poor outcomes, dabrafenib monotherapy and combination dabrafenib plus trametinib should be considered in the management of patients with NSCLC harboring BRAF V600E mutations. Areas for future research remain and include direct head-to-head comparisons of BRAF monotherapy and combination BRAF-MEK inhibition, long-term follow-up of the safety profile of these targeted therapies, evaluation of the efficacy of dabrafenib and trametinib in the first-line treatment setting, and explorations of treatment options for patients with tumors harboring less common BRAF mutations.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Shaohua Cui (Department of Pulmonary Medicine, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.11.50). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. Version 6. 2015.
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949-54. [Crossref] [PubMed]
- Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol 2011;29:2046-51. [Crossref] [PubMed]
- Marchetti A, Felicioni L, Malatesta S, et al. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J Clin Oncol 2011;29:3574-9. [Crossref] [PubMed]
- Cardarella S, Ogino A, Nishino M, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res 2013;19:4532-40. [Crossref] [PubMed]
- Tissot C, Couraud S, Tanguy R, et al. Clinical characteristics and outcome of patients with lung cancer harboring BRAF mutations. Lung Cancer 2016;91:23-8. [Crossref] [PubMed]
- Villaruz LC, Socinski MA, Abberbock S, et al. Clinicopathologic features and outcomes of patients with lung adenocarcinomas harboring BRAF mutations in the Lung Cancer Mutation Consortium. Cancer 2015;121:448-56. [Crossref] [PubMed]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16. [Crossref] [PubMed]
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380:358-65. [Crossref] [PubMed]
- Gautschi O, Milia J, Cabarrou B, et al. Targeted Therapy for Patients with BRAF-Mutant Lung Cancer: Results from the European EURAF Cohort. J Thorac Oncol 2015;10:1451-7. [Crossref] [PubMed]
- Liu W, Kelly JW, Trivett M, et al. Distinct clinical and pathological features are associated with the BRAF(T1799A(V600E)) mutation in primary melanoma. J Invest Dermatol 2007;127:900-5. [Crossref] [PubMed]
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol 2011;29:1239-46. [Crossref] [PubMed]
- Planchard D, Kim TM, Mazieres J, et al. Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol 2016;17:642-50. [Crossref] [PubMed]
- Planchard D, Besse B, Groen HJ, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 2016;17:984-93. [Crossref] [PubMed]
- Gautschi O, Pauli C, Strobel K, et al. A patient with BRAF V600E lung adenocarcinoma responding to vemurafenib. J Thorac Oncol 2012;7:e23-4. [Crossref] [PubMed]
- Peters S, Michielin O, Zimmermann S. Dramatic response induced by vemurafenib in a BRAF V600E-mutated lung adenocarcinoma. J Clin Oncol 2013;31:e341-4. [Crossref] [PubMed]
- Robinson SD, O'Shaughnessy JA, Cowey CL, et al. BRAF V600E-mutated lung adenocarcinoma with metastases to the brain responding to treatment with vemurafenib. Lung Cancer 2014;85:326-30. [Crossref] [PubMed]
- Schmid S, Siano M, Joerger M, et al. Response to dabrafenib after progression on vemurafenib in a patient with advanced BRAF V600E-mutant bronchial adenocarcinoma. Lung Cancer 2015;87:85-7. [Crossref] [PubMed]
- Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet 2012;379:1893-901. [Crossref] [PubMed]
- Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N Engl J Med 2015;373:726-36. [Crossref] [PubMed]
- Lopez-Chavez A, Thomas A, Rajan A, et al. Molecular profiling and targeted therapy for advanced thoracic malignancies: a biomarker-derived, multiarm, multihistology phase II basket trial. J Clin Oncol 2015;33:1000-7. [Crossref] [PubMed]
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998-2006. [Crossref] [PubMed]
- Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016;387:1415-26. [Crossref] [PubMed]
- Goldman JM, Gray JE. BRAF V600E mutations: a series of case reports in patients with non-small cell lung cancer. Cancer Genet 2015;208:351-4. [Crossref] [PubMed]
- Myall NJ, Neal JW, Cho-Phan CD, et al. Long-Term Survival of a Patient With Non-Small-Cell Lung Cancer Harboring a V600E Mutation in the BRAF Oncogene. Clin Lung Cancer 2016;17:e17 [Crossref] [PubMed]
- Crinò L, Mosconi AM, Scagliotti G, et al. Gemcitabine as second-line treatment for advanced non-small-cell lung cancer: A phase II trial. J Clin Oncol 1999;17:2081-5. [PubMed]
- van Putten JW, Baas P, Codrington H, et al. Activity of single-agent gemcitabine as second-line treatment after previous chemotherapy or radiotherapy in advanced non-small-cell lung cancer. Lung Cancer 2001;33:289-98. [Crossref] [PubMed]
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000;18:2095-103. [PubMed]
- Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol 2000;18:2354-62. [PubMed]
- Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384:665-73. [Crossref] [PubMed]
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97. [Crossref] [PubMed]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [Crossref] [PubMed]
- Karampeazis A, Voutsina A, Souglakos J, et al. Pemetrexed versus erlotinib in pretreated patients with advanced non-small cell lung cancer: a Hellenic Oncology Research Group (HORG) randomized phase 3 study. Cancer 2013;119:2754-64. [Crossref] [PubMed]
- Garassino MC, Martelli O, Broggini M, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol 2013;14:981-8. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Montagut C, Sharma SV, Shioda T, et al. Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res 2008;68:4853-61. [Crossref] [PubMed]
- Johannessen CM, Boehm JS, Kim SY, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 2010;468:968-72. [Crossref] [PubMed]
- Nazarian R, Shi H, Wang Q, Kong X, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 2010;468:973-7. [Crossref] [PubMed]
- Wagle N, Emery C, Berger MF, et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol 2011;29:3085-96. [Crossref] [PubMed]
- Trunzer K, Pavlick AC, Schuchter L, et al. Pharmacodynamic effects and mechanisms of resistance to vemurafenib in patients with metastatic melanoma. J Clin Oncol 2013;31:1767-74. [Crossref] [PubMed]
- Sabbatino F, Wang Y, Wang X, et al. PDGFRα up-regulation mediated by sonic hedgehog pathway activation leads to BRAF inhibitor resistance in melanoma cells with BRAF mutation. Oncotarget 2014;5:1926-41. [Crossref] [PubMed]
- Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014;371:1877-88. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Douillard JY, Ostoros G, Cobo M, et al. First-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: a phase-IV, open-label, single-arm study. Br J Cancer 2014;110:55-62. [Crossref] [PubMed]
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [Crossref] [PubMed]
- Lee le M. Feun L, Tan Y. A case of intracranial hemorrhage caused by combined dabrafenib and trametinib therapy for metastatic melanoma. Am J Case Rep 2014;15:441-3. [Crossref] [PubMed]


