
Prostaglandin E2 plays a major role in glioma resistance and progression
Glioblastoma multiforme (GBM) is the most common primary malignant tumor of the central nervous system in adults. The prognosis for these patients remains extremely poor despite aggressive therapy including maximal surgical resection followed by radiation plus concomitant and adjuvant chemotherapy in the form of temozolomide (1). In spite of this intense therapy most patients will recur within about 7 months after the initial diagnosis and usually in or around the original site. Many studies have linked the poor response of GBM to treatment, to the presence of cancer stem/initiator cells (CSC) that are highly resistant to chemical agents and radiation because of their inability to undergo cell death (2).
Recent studies have pointed out the bioactive lipid prostaglandin E2 (PGE2) as a major actor in cancers including gliomas (3,4). PGE2 belongs to the prostanoid family of lipids, which is a subclass of eicosanoids produced by oxidation of phospho-membrane lipids including arachidonic acid (AA) that is converted into PGH2 by COX enzymes. There are two major forms of COX: COX-1 and COX-2, which is an immediate early response gene that is normally absent from most cells but is highly induced at sites of inflammation and during tumor progression (5). Several studies have demonstrated that COX-2 is over-expressed in gliomas and could be positively correlated with tumor grade. Indeed, elevated COX-2 levels correlate with earlier recurrence and shorter survival in glioma patients (5,6). COX-2, which is constitutively expressed in the central nervous system is central in the synthesis of prostanoids (7). The conversion of PGH2 to PGE2 is mediated by prostaglandin E synthase (PGES) that exists in three isoforms: membrane associated PGES-1 (mPGES-1), mPGES-2 and cytoplasmic PGES (cPGES). mPGES-1 is functionally associated with COX-2 and rapidly induced by various stimuli to generate a peak in PGH2. In glioma, the overexpression of mPGES-1 and its link with enhanced apoptosis have been positively correlated to the survival of patients (3).
However, it has to be kept in mind that PGH2 is associated with a broad range of biological effects and fulfills complex (and sometimes antagonistic) roles under both normal and pathological situations. The localization and partners of PGH2 are important. For example, PGE2 is pro-apoptotic when intracellular and anti-apoptotic when it is released from the cells (8). The observation that PGE2 has potent tumor-promoting activity is centered on the considerable body of evidence obtained from rodent studies as well as research on the protective effects of non-steroidal anti-inflammatory drugs (NSAIDs) in cancer risks (9).
PGE2 binds to members of the EP family of receptors that consists of four isoforms (EP1–4). These EP receptors are coupled to Gα proteins that contain stimulatory (GαS) or inhibitory (GαI) subunits that can modulate the levels of Ca2+, cAMP and inositol phosphate thereby activating divergent downstream signaling pathways accounting for the pleiotropic actions of PGE2 in proliferation, apoptosis, angiogenesis inflammation and immune surveillance (see Figure 1). In fact, mPGES-1 was found to be higher in recurrent grade II gliomas (10). Significant crosstalk exists between the EP1, EP2 and EP4 receptors and the epidermal growth factor receptor (EGFR) signaling pathway through subsequent transactivation of the EGFR, which results in the activation of c-Src (see Figure 1). Activation of EGFR leads to the activation of several signal transduction cascades, engaging mitogen-activated protein kinase (MAPK), PI3K/Akt, STAT and phospholipase C (PLC) signaling pathways, culminating in cell proliferation, differentiation or migration.
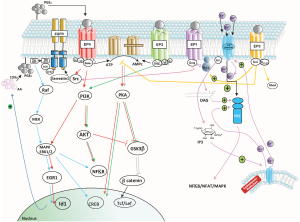
In the article by Cook et al. (11) glioma cells obtained from a PDGF-mouse brain induced tumor using the RCAS-tva system were used to investigated the functional role of PGE2 signaling in GBM and confirmed the over-expression of COX-2 and PGE2. These authors underline the importance of EP2 and EP4 receptors, especially EP4.
Activation of the EP2 receptor causes the activation of several signaling pathways including activation of protein kinase A (PKA) that is responsible for activating the transcription factor cyclic adenosine monophosphate response-element binding protein (CREB) and the signaling factor extracellular signal-regulated kinase-1/2 (ERK1/2). Activation of EP2 can also lead to the formation of a β-arrestin 1-Src complex, with subsequent activation of the EGFR. Activation of the EP4 receptor triggers the same signaling pathway through activation of the transcription factor CREB, but also initiates activation of the PI3K and AKT pathway. As such, the biological outcomes of EP2 vs. EP4 activation are somewhat different (12). The EP1/EP1 receptor, which was shown to signal through a PLC/PKC/c-Src signaling pathway, is also involved in PGE2-mediated GBM proliferation, and EP1 antagonists have been shown to inhibit the proliferation of glioma cell lines in vitro and slow tumor growth in vivo (13). However, the article of Cook et al. present the EP4 receptor as distinctively responsible for PGE2-mediated induction of inhibitor of DNA binding 1 (Id1) and demonstrate the importance of the MAPK signal required for PGE2-mediated induction of Id1 in GBM cells. The EP1 and EP2 receptors are low affinity receptors, compared to EP3 and EP4 and as such should be activated at low concentrations of PGE2, but the specific connection of EP4 (and not EP2) in Id1 activation is established here. Additional survival/proliferation mechanisms by PGE2 have been shown by Brocard et al. (14) who demonstrated that in vitro radiation-induced PGE2 sustained human glioma cell proliferation and survival through EGFR signaling by PGE2 mainly through EP2.
Another important point underlined in the article is the impact of the activation of Id1 on glioma cell proliferation and the self-renewal of the glioma stem cells. High Id1 levels often correlate strongly with poor prognosis, therapeutic resistance, tumor metastases and a high self-renewal of cancer stem cells. However, in another PDGF-driven tumor model, Id1low cells were shown to generate tumors more quickly and with higher infiltration than Id1high cells (15). Subsequently, targeting the Cox-2/Id1 pathway to eliminate glioma stem cells may not necessary improve the outcome of patient with GBM and it could depend on several/other factors including the glioma subgroup. Similarly, an analyses of transcriptomic results showed that Id1high expressing patients demonstrated a better overall survival compared to Id1low expressing patients suggesting that high levels of Id1 are a good prognostic factor (16). Essentially Id1 was shown to affect the efficacy of radiotherapy in GBM initiating an accumulation of cells in G2/M and a subsequent diminution in DNA repair. This observation is particularly interesting, as the main functions of Id1–Id4 have been shown to inhibit differentiation and promote proliferation of many different cell types. However, in the brain, the expression of Id2 has been shown to be associated with cultured neural precursor cell (NPC) proliferation as well as with the proliferation of central nervous system tumors including glioblastoma (17). These results point out that the Id1/Id2 ratio could be a determinant in the control of glioma growth.
Radiotherapy remains the major treatment for GBM, the therapeutic consequence of which is the eradication of tumor cells and damage to surrounding normal tissue. However, as stated above, there is a rapid recurrence probably due to the presence of the CSC, which are radio-resistant. These CSC in the damaged tissue play a critical role in tissue regeneration and tumor recurrence. It is generally assumed that in dying cells caspase-activated pathways would release growth-promoting factors that mobilize and recruit CSC. One of these pathways is the Ca2+-independent phospholipase A2, the activation of which increases the synthesis and release of AA from apoptotic cells and the consequent release of PGE2 by these dying cells. As such, this radiation-induced release of PGE2 would constitute the “dark side” of radiotherapy that has to be counteracted to inhibit tumor growth (18). The effect of apoptotic cells on the surrounding tissue cancer cell proliferation may be driven by inappropriate signals from these cells and modify the impact of apoptosis on tumorigenesis and cancer therapy.
It has already been described that COX-2 derived-PGE2 could induce Id1 thereby increasing CSC self-renewal and radiation resistance. Actually, numerous studies have demonstrated that supplementing with exogenous PGE2 recapitulates radio-resistance, thus blocking the EP2 receptor would abrogate radio-resistance (13,19). In another study it was demonstrated that radiotherapy induces a caspase-3-dependent mechanism involving PGE2 and was responsible for the regeneration of surviving cells (20). Likewise, the role of Id1 in glioma tumorigenesis through the COX-2-PGE2 pathway and role of Id1 in the proliferation of stem cells after radiation has been characterized (6).
Overall, the results suggest that this topic is essential in the case of fractionation radiation doses associated with chemotherapy in the treatment of GBM. If previous studies have demonstrated that CSC are selectively enriched after chemotherapy through enhanced survival and chemo-resistance. These results confirm the negative aspects of the induction of apoptosis in cancer cells by radiotherapy in glioma treatment. However, because members of the prostaglandin family have pleiotropic and often contradictory roles, the efficient targeting of the synthesis of PGE2 by inhibitors of mPGES or the modulation of the activity of its receptors (EP2 and EP4 especially) seems to be attractive strategies.
Future investigations are needed to identify the groups of patients, defined by genomic characteristics among others that could benefit from this approach in GBM. In addition the Id1/Id2 expression could be good candidates as biomarkers to determine the tumors that would respond to these treatments.
Acknowledgments
The authors wish to apologize for many fine works on this topic not cited in this editorial.
Funding: The work in our group is supported by the “Hospital University Centre of Nantes”, the INSERM and the Faculty of Pharmacy at the University of Nantes, France.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Hongcheng Zhu, MD, PhD (Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical university, Nanjing, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.11.20). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96. [Crossref] [PubMed]
- Hambardzumyan D, Becher OJ, Holland EC. Cancer stem cells and survival pathways. Cell Cycle 2008;7:1371-8. [Crossref] [PubMed]
- Lalier L, Cartron PF, Pedelaborde F, et al. Increase in PGE2 biosynthesis induces a Bax dependent apoptosis correlated to patients' survival in glioblastoma multiforme. Oncogene 2007;26:4999-5009. [Crossref] [PubMed]
- Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer 2010;10:181-93. [Crossref] [PubMed]
- Shono T, Tofilon PJ, Bruner JM, et al. Cyclooxygenase-2 expression in human gliomas: prognostic significance and molecular correlations. Cancer Res 2001;61:4375-81. [PubMed]
- Xu K, Wang L, Shu HK. COX-2 overexpression increases malignant potential of human glioma cells through Id1. Oncotarget 2014;5:1241-52. [Crossref] [PubMed]
- Patrignani P, Tacconelli S, Sciulli MG, et al. New insights into COX-2 biology and inhibition. Brain Res Brain Res Rev 2005;48:352-9. [Crossref] [PubMed]
- Mignard V, Lalier L, Paris F, et al. Bioactive lipids and the control of Bax pro-apoptotic activity. Cell Death Dis 2014;5:e1266 [Crossref] [PubMed]
- Fischer SM, Hawk ET, Lubet RA. Coxibs and other nonsteroidal anti-inflammatory drugs in animal models of cancer chemoprevention. Cancer Prev Res (Phila) 2011;4:1728-35. [Crossref] [PubMed]
- Mattila S, Tuominen H, Koivukangas J, et al. The terminal prostaglandin synthases mPGES-1, mPGES-2, and cPGES are all overexpressed in human gliomas. Neuropathology 2009;29:156-65. [Crossref] [PubMed]
- Cook PJ, Thomas R, Kingsley PJ, et al. Cox-2-derived PGE2 induces Id1-dependent radiation resistance and self-renewal in experimental glioblastoma. Neuro Oncol 2016;18:1379-89. [Crossref] [PubMed]
- Rundhaug JE, Simper MS, Surh I, et al. The role of the EP receptors for prostaglandin E2 in skin and skin cancer. Cancer Metastasis Rev 2011;30:465-80. [Crossref] [PubMed]
- Matsuo M, Yoshida N, Zaitsu M, et al. Inhibition of human glioma cell growth by a PHS-2 inhibitor, NS398, and a prostaglandin E receptor subtype EP1-selective antagonist, SC51089. J Neurooncol 2004;66:285-92. [Crossref] [PubMed]
- Brocard E, Oizel K, Lalier L, et al. Radiation-induced PGE2 sustains human glioma cells growth and survival through EGF signaling. Oncotarget 2015;6:6840-9. [Crossref] [PubMed]
- Barrett LE, Granot Z, Coker C, et al. Self-renewal does not predict tumor growth potential in mouse models of high-grade glioma. Cancer Cell 2012;21:11-24. [Crossref] [PubMed]
- Guo Q, Guo P, Mao Q, et al. ID1 affects the efficacy of radiotherapy in glioblastoma through inhibition of DNA repair pathways. Med Oncol 2013;30:325. [Crossref] [PubMed]
- Sullivan JM, Havrda MC, Kettenbach AN, et al. Phosphorylation Regulates Id2 Degradation and Mediates the Proliferation of Neural Precursor Cells. Stem Cells 2016;34:1321-31. [Crossref] [PubMed]
- Kurtova AV, Xiao J, Mo Q, et al. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature 2015;517:209-13. [Crossref] [PubMed]
- Huang X, Taeb S, Jahangiri S, et al. miR-620 promotes tumor radioresistance by targeting 15-hydroxyprostaglandin dehydrogenase (HPGD). Oncotarget 2015;6:22439-51. [Crossref] [PubMed]
- Huang Q, Li F, Liu X, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med 2011;17:860-6. [Crossref] [PubMed]


