
Treatment of recurrent epithelial ovarian carcinoma with single-agent apatinib
Introduction
Epithelial ovarian carcinoma (EOC) is the leading cause of death from gynecological cancers in women (1). An estimated up to 85% of patients with EOC will develop recurrent disease within 2 years even after radical surgery and combination chemotherapy (2). After recurrence, response rates to second-line chemotherapy for platinum-sensitive patients are approximately 30%, while for those, with platinum-resistant disease are only 10–25% to chemotherapeutic agents (3). The overall survival rate at 5 years is 50% and the 75% of patients die of recurrent disease (4,5). Thus, EOC is as regarded as a clinically incurable disease. Novel approaches with biologic agents that target the mechanisms of tumor growth and metastasis are needed.
Among the various factors contributing to tumor progression, angiogenesis plays an important role, as it helps tumor growth by ensuring sufficient oxygen and nutrient supply required for unlimited proliferation of tumor cells and growth of new blood vessels, and furthermore potentially results in tumor progression and metastasis (6). Basically, the direct interaction between tissue vascular endothelial growth factor receptors (VEGFRs) and their soluble ligands (VEGFs) mainly regulates the procedures of angiogenesis (7). The VEGFR family involves three kinds of type II transmembrane proteins (VEGFR-1, VEGFR-2 and VEGFR-3), which are characterized by a tyrosine kinase activity (8). Among these receptors, VEGFR-2 is the most important mediator of the VEGF-induced angiogenic signaling (8).
Apatinib (YN968D1) is a novel and strongly selective VEGFR-2 tyrosine kinase inhibitor (TKI) that targets the intracellular ATP binding site of the receptor, and prevents phosphorylation and subsequent downstream signaling (9). Here, we report a unique case of recurrent EOC, where the patient showed significant response to apatinib following the failure to chemotherapy.
Case presentation
A 59-year-old Chinese woman was admitted to our department with a 1-month history of irregular postmenopausal bleeding on January 7th 2015. The patient did not display any remarkable abnormality after physical examination, and had no history of hypertension, cardiac diseases, gastritis, gastric ulcer or nephritis. The ultrasound examination showed a 4.2 cm × 3.4 cm solid mass in the left adnexa uterus and a 5.7 cm × 4.0 cm one in the right adnexa uterus. A MRI scan revealed enlarged lymph nodes in the pelvic cavity. The serum levels of tumor markers were as follows: CA125, 2,871.00 U/mL, and ROMA index (postmenopausal), 98.61%. At the time of admission, the patient was diagnosed of postmenopausal bleeding, and the possibility of endometrium carcinoma, or ovarian carcinoma in both adnexa uteri.
Later on January 9th, a diagnostic curettage was performed and the pathological examination suggested breaks in the endometrium and proliferation stage in mucus. This result excluded endometrium carcinoma and indicated towards the likely possibility of the diagnosis of ovarian carcinoma.
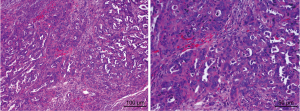
Subsequently, after written informed consent was obtained from the patient, an operation incorporating of radical hysterectomy (removing the whole uterus, tissue on the sides of the uterus, the cervix, and 2–3 cm of the top part of the vagina), bilateral adnexectomy, omentectomy, appendectomy and metastasectomy of liver, diaphragm and peritoneum were performed on January 18th. Intra-operatively, a friable cauliflower-like 4.0 cm × 5.0 cm × 5.0 cm mass adhering to the left fallopian tube and ovary was observed, while the right ovary appeared normal in appearance. However, the right fallopian tube was about 2 cm thick, and displayed cauliflower-like tissue on its surface. Bilateral adnexa uteri were densely adhered to the pelvic wall and rectum, and cauliflower-like tissues were scattered on the pelvic peritoneal, but no ascites within the peritoneal cavity were observed. In addition, multiple metastatic masses of size of 2.0 cm × 2.0 cm on the surface of Glisson’s capsule and diaphragm were observed. A metastatic mass in the size of 1.0 cm × 1.0 cm was also noticed in the ligamentum teres hepatic. The multiple metastatic masses of size 3.0 cm × 4.0 cm × 5.0 cm touching the surface of greater omentum were also observed. The resected masses from the pelvic cavity were sent for rapid pathological examination and led to confirmation of adenocarcinoma. The ovarian papillary serous carcinoma was confirmed based on the examination of intraoperative frozen sections (Figure 1). This poor differentiated carcinoma involved the serous surface of uterus and the right fallopian tube and surrounding fat. The metastatic masses in the pelvic cavity and surface of liver, diaphragm and sigmoid colon were confirmed to be papillary adenocarcinomas. Tumor cells were found in celiac metastases, ligamentum teres hepatic and greater omentum, but not in the ascites. Tumor thrombi were noticed in the vessels. These observations led to the diagnosis of papillary serous ovarian carcinoma of stage IIIC.
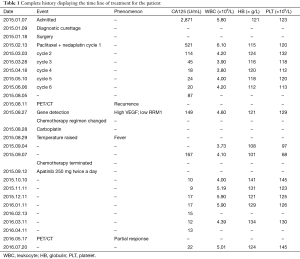
After this diagnosis, the patient received six cycles of chemotherapy involving the regimen of paclitaxel 210 mg on day 1 + nedaplatin 120 mg on day 2 on February 13th, March 3rd, March 28th, April 18th, May 10th and June 6th, respectively. The patient tolerated the chemotherapy well and the results of blood routine test, liver function and tumor markers were listed in Table 1.
Full table
Next, on August 5th, 2 months after the end of the first-line chemotherapy, the serum CA125 level of increased to 87.39 U/mL. In addition, a PET/CT scan on August 11th revealed enhanced uptake of 18F-labeled fluorodeoxyglucose (FDG) in the anterior median peritoneotome of upper abdomen, the left lobe and caudate lobe of the liver, splenic hilus and inside the spleen. This observation led to the diagnosis of recurrent ovarian carcinoma with extensive metastases into the abdominal cavity.
At this stage, multidisciplinary consultation involving specialists from oncology, interventional therapy and hepatobiliary surgery was scheduled. As the tumor had metastasized within 2 months after the six cycles of standard postoperative chemotherapy, oncologists suggested that the metastases were due to drug resistance and the summary of the advices from specialists included (I) interventional ablation; (II) resection of the spleen and metastases in the liver; (III) molecular targeted therapy; or (IV) chemotherapy with different regimens. Based on the general status of the patient the adopted treatment modality was decided to be second-line chemotherapy or molecular targeted therapy and followed by alternative surgery or interventional ablation.
In parallel, on August 27th, the levels of VEGF and RRM1 proteins were analyzed the results revealed high expression of VEGF protein and low expression of RRM1 protein, therefore suggesting that the patient was sensitive to apatinib. On the same day, the CA125 level elevated to 149 U/mL.
Meanwhile, the patient was willing and already scheduled to receive chemotherapy with the changed regimen including carboplatin 0.4 g day 1 plus gemcitabine 1,400 mg day 2. Thus, on August 28th, the carboplatin 0.4 g was administered, but the next day the patient developed fever with a peak of 38.2 °C and so the administration of gemcitabine was suspended. The blood routine tests on the following days displayed that levels of platelets, leukocytes and hemoglobin were progressively decreasing, with a minimum levels of 68×109/L, 101×109/L and 3.73 g/L, respectively. These results indicated serious bone marrow depression due to chemotherapy. Moreover, the serum level of CA125 on September 7th rose to 167 U/mL. Therefore, it was decided to terminate the administration of chemotherapy.
At this stage, after receiving the written consent from the patient, apatinib mesylate tablets (Chinese name Aitan) were administered at a dose of 250 mg twice a day after meals, starting on September 12th. On September 22th, the patient developed hypertension (range of 145–160/95–100 mmHg), along with anorexia, nausea and vomiting. In addition, on September 27th, dental ulcer and throat pain were also noticed, and by October 2nd the patient had complaints about abdominal pain and diarrhea. Then a gastroscopy revealed gastric ulcer. Besides, the patient was diagnosed of hand-foot syndrome on October 9th. But all these side effects or complaints were relieved or disappeared after symptomatic treatments.
Interestingly, the blood analysis of the results including BRT, liver functions and CA125 levels on October 10th, 27th and November 11th displayed normal. Specifically, the CA125 serum levels decreased to 10 U/mL and since then maintained normal. The ultrasound examination on November 11th did not identify any abnormality. The results of liver functions test on November 27th revealed ALT value of 111 U/L and AST value of 128 U/L, respectively. Thus, the administration of apatinib was discontinued. After hepatoprotective management until the liver function recovered to normal range, the administration of apatinib was continued at the same dose as previously. The December 27th CT scan revealed no abnormality in the former bilateral adnexa areas.
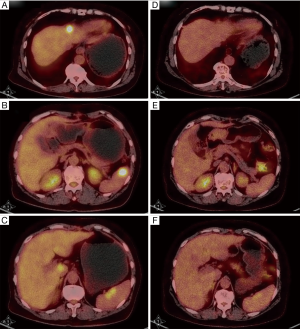
Finally on May 17th 2016, a PET/CT scan showed no enhanced FDG uptake in the abdomen or pelvic cavity (Figure 2). Moreover, the enhanced FDG uptake observed in the anterior median peritoneotome of upper abdomen, the left lobe and caudate lobe of the liver, splenic hilus and inside the spleen also disappeared when checked on August 11th, 2015. At this time, we accessed the state of disease and concluded that the patient had a partial remission. Since then regular CA125 tests and BRTs showed normal results, with the latest CA125 test on July 20th showing a value of 22.71 U/mL (Table 1).
Discussion
This is the first report of a patient with recurrent EOC, responding positively to apatinib, a VEGFR-2 TKI. So far there has not been any similar report in the literature. This report highlights the experience of clinical physicians, while using apatinib in clinical practice.
EOC patients are usually expected to respond to initial chemotherapy, however, they often develop resistance to platinum-based regimens. The patient in our report also displayed similar characteristic. The antitumor effect mechanism of apatinib does not involve the inhibition of in vitro growth (cell proliferation) of cancer cell, but is mediated by its antiangiogenic role, which is different from the functional mechanism of chemotherapeutic agents (10).
Angiogenesis has been a crucial step for tumor cell survival, proliferation, local invasion, and distant metastasis (11). Overexpression of VEGF and VEGFR correlates with increased tumor cell growth rate, microvessel density, proliferation, metastatic potential and poor prognosis in multiple cancers (12). Therefore, inhibition of VEGFR signaling is a promising therapeutic target, and involves blockage of VEGF/VEGFR pathway through specific inhibitors (antibodies or small molecules), either binding to VEGF or interfering with certain domains of VEGFR. Recently, many VEGFR-2 inhibitors such as sorafenib, vandetanib, cediranib, and sunitinib have been developed (13-15).
Besides, apatinib can also effectively inhibit, to some extent, the activities of platelet-derived growth factor-b, c-kit and c-src proteins, all of which have been implicated in the pathogenesis of malignant tumors (16-18). Apatinib has also been shown to reverse the P-glycoprotein (ABCB1)- and ABCG2-mediated multidrug resistance in drug-resistant solid tumor cells by inhibiting their transport function (19). In addition, apatinib can also block the formation of rat aortic ring which mimics the multiple steps of in vitro angiogenesis (10). Based on these mechanisms, apatinib inhibits VEGF-stimulated endothelial cell migration and proliferation and subsequently not only decrease the tumor microvascular density (9), but also is able to circumvent cancer cell resistance to other antineoplastic agents. In the present case, apatinib exhibited its antitumor ability after the patient was resistant to the drugs in the first-line chemotherapy.
As a compound derived from valatinib, apatinib has shown superior efficacy in xenograft study (20). Also, its in vitro IC50 value for 50% enzymatic inhibition has been lower than other recently-developed anti-VEGFR agents such as sunitinib (0.001 vs. 0.005, respectively) (10,21).
The antitumor activity of apatinib has been validated in several preclinical tumor models (10), while its clinical efficacy has been demonstrated in patients with gastric cancer (22,23), hepatic cancer (24), breast cancer (24) and colorectal cancer (9). After its approval by China Food and Drug Administration, apatinib has been suggested to treat chemotherapy-refractory advanced gastric cancer and adenocarcinoma of the gastroesophageal junction for Chinese patients.
The pharmacokinetic and dose-escalation results indicate that apatinib is well tolerated by most patients at a daily dose of ≤850 mg (9). The results from a phase I clinical study showed that apatinib exhibits substantial antitumor activity across many malignancies at the recommended dose of 750 mg once daily (9). However, significant inter-patient variability with apatinib warrants dose modification to meet individual needs. Most side effects with dose of 500 mg/day were mild to moderate (25). For patients with non-small cell lung cancer, Zhang et al. (26) and Hu et al. (25) recommended an initial dose to be 500 mg rather than the originally reported 750 mg for treating other malignancies. In this report, the patient received a dose of 500 mg/day.
In generally, the toxicity of apatinib was controllable and tolerable and most of the side effects were caused by the inhibition of blood vessel endothelia. Systemic hypertension is a common side effect and can be attributed to the inhibition of VEGFR in arterial endothelial cells, and decrease in the release of nitric oxide, which then acts on arterial smooth muscle cells to cause vasodilation (27). Hypertension has been observed with all of the oral VEGF TKIs, as reported previously (28), and therefore, patients with preexisting hypertension should be evaluated cautiously for receiving apatinib, due to safety issues. If serious side effects, such as gastrointestinal perforation, urgent wound dehiscence, fistula, severe hemorrhage, nephritic syndrome or hypertensive crisis are observed, the apatinib administration should be immediately discontinued. So far, no study has reported a correlation between the appearance of side effects, as possible surrogate markers, and the response to apatinib.
It is important to have suitable biomarkers to predict the efficacy of antiangiogenic agents. A biomarker study of apatinib in patients with breast cancer showed that both hypertension and high expression of phosphorylated VEGFR-2 could serve as potential biomarkers for its efficacy (29).
CA125 is the tumor marker often considered as the “gold standard” in ovarian cancer (30) and can be measured with a simple blood test. The CA125 level has been shown to increase in majority of the EOC patients with EOC (9) and correlate with the course of the disease (10). Numerous studies have confirmed the usefulness of CA125 levels in monitoring the progress of patients with epithelial ovarian cancer (31-34). Commonly accepted definitions of disease recurrence based on serum CA125 levels alone specify a doubling of this tumor marker level, either from the upper limit of normal (35 U/mL) in patients with normalization of this marker after primary treatment or from the nadir levels in patients with an elevated serum marker value that never normalizes after primary treatment (35,36). Most reports indicate that a rise in CA125 levels precedes clinical detection by about 3 months (37). In our case, the CA125 level decreased to normal range under chemotherapy, indicating that our case was sensitive to CA125. And the elevation of CA125 value after the first-line chemotherapy was concurrent with the recurrence of disease. Besides, a PET/CT scan also revealed enhanced uptake of 18F-labeled FDG in multiple sites. Thus, the diagnosis of recurrent disease was made.
PET/CT has the better efficiency at detecting recurrent smaller tumors, thus allowing earlier detection particularly when the only evidence of recurrence is a steadily increasing serum CA125 level (38). Previous studies have demonstrated the ability of PET/CT to diagnose tumor recurrence even when the serum CA125 level is <30 U/mL or peritoneal implants are smaller than 2 cm. Generally, PET/CT has much better overall sensitivity, specificity, accuracy and positive- and negative-predictive values than CT or MRI in the detection of recurrent EOC. That is the reason why we selected PET/CT as an evaluation method.
In conclusion, recurrent EOC is probably incurable, and its management remains a challenge for clinical practitioners. However, in well-selected patients with recurrent EOC, apatinib may contribute to achieve clinical benefits with an acceptable safety profile. However, the definite efficacy of apatinib and the correct sequence of the treatment warrant further evaluation involving large-scale clinical trials.
Acknowledgments
Funding: This work was supported by grants from Society Development Plans, Department of Science and Technology Changzhou (CJ20159018).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.01.01). The authors have no conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Gardner GJ, Jewell EL. Current and future directions of clinical trials for ovarian cancer. Cancer Control 2011;18:44-51. [PubMed]
- Vargas-Hernández VM, Moreno-Eutimio MA, Acosta-Altamirano G, et al. Management of recurrent epithelial ovarian cancer. Gland Surg 2014;3:198-202. [PubMed]
- Wu MD, Wang Y, Ding T, et al. A retrospective clinical study of bevacizumab combined with gemcibabine or paclitaxel in the treatment of recurrent ovarian cancer. Indian J Cancer 2014;51:e103-5. [Crossref] [PubMed]
- Rubin SC, Randall TC, Armstrong KA, et al. Ten-year follow-up of ovarian cancer patients after second-look laparotomy with negative findings. Obstet Gynecol 1999;93:21-4. [PubMed]
- Sullivan LA, Brekken RA. The VEGF family in cancer and antibody-based strategies for their inhibition. MAbs 2010;2:165-75. [Crossref] [PubMed]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669-76. [Crossref] [PubMed]
- Fontanella C, Ongaro E, Bolzonello S, et al. Clinical advances in the development of novel VEGFR2 inhibitors. Ann Transl Med 2014;2:123. [PubMed]
- Li J, Zhao X, Chen L, et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer 2010;10:529. [Crossref] [PubMed]
- Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci 2011;102:1374-80. [Crossref] [PubMed]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000;407:249-57. [Crossref] [PubMed]
- Longo R, Gasparini G. Challenges for patient selection with VEGF inhibitors. Cancer Chemother Pharmacol 2007;60:151-70. [Crossref] [PubMed]
- Wood JM, Bold G, Buchdunger E, et al. PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res 2000;60:2178-89. [PubMed]
- Clark JW, Eder JP, Ryan D, et al. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors. Clin Cancer Res 2005;11:5472-80. [Crossref] [PubMed]
- Wedge SR, Ogilvie DJ, Dukes M, et al. ZD4190: an orally active inhibitor of vascular endothelial growth factor signaling with broad-spectrum antitumor efficacy. Cancer Res 2000;60:970-5. [PubMed]
- Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene 2000;19:5636-42. [Crossref] [PubMed]
- Heinrich MC, Blanke CD, Druker BJ, et al. Inhibition of KIT tyrosine kinase activity: a novel molecular approach to the treatment of KIT-positive malignancies. J Clin Oncol 2002;20:1692-703. [Crossref] [PubMed]
- Song S, Ewald AJ, Stallcup W, et al. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol 2005;7:870-9. [Crossref] [PubMed]
- Mi YJ, Liang YJ, Huang HB, et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res 2010;70:7981-91. [Crossref] [PubMed]
- Chen P, Iruela-Arispe L, Lou L, et al. VEGFr inhibitor YN968D1 xenograft dose response studies against human colon cancer Ls174t and HT29. Proc Amer Assoc Cancer Res 2006;47:abstr 1764.
- Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 2003;9:327-37. [PubMed]
- Roviello G, Ravelli A, Polom K, et al. Apatinib: A novel receptor tyrosine kinase inhibitor for the treatment of gastric cancer. Cancer Lett 2016;372:187-91. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 2013;31:3219-25. [Crossref] [PubMed]
- Geng R, Li J. Apatinib for the treatment of gastric cancer. Expert Opin Pharmacother 2015;16:117-22. [Crossref] [PubMed]
- Hu X, Zhang J, Xu B, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer 2014;135:1961-9. [Crossref] [PubMed]
- Zhang L, Shi M, Huang C, et al. A phase II, multicenter, placebo-controlled trial of apatinib in patients with advanced nonsquamous non-small cell lung cancer (NSCLC) after two previous treatment regimens. J Clin Oncol 2012;30 suppl; abstr 7548.
- Tang JR, Markham NE, Lin YJ, et al. Inhaled nitric oxide attenuates pulmonary hypertension and improves lung growth in infant rats after neonatal treatment with a VEGF receptor inhibitor. Am J Physiol Lung Cell Mol Physiol 2004;287:L344-51. [Crossref] [PubMed]
- Izzedine H, Rixe O, Billemont B, et al. Angiogenesis inhibitor therapies: focus on kidney toxicity and hypertension. Am J Kidney Dis 2007;50:203-18. [Crossref] [PubMed]
- Fan M, Zhang J, Wang Z, et al. Phosphorylated VEGFR2 and hypertension: potential biomarkers to indicate VEGF-dependency of advanced breast cancer in anti-angiogenic therapy. Breast Cancer Res Treat 2014;143:141-51. [Crossref] [PubMed]
- Høgdall E. Cancer antigen 125 and prognosis. Curr Opin Obstet Gynecol 2008;20:4-8. [Crossref] [PubMed]
- Meyer T, Rustin GJ. Role of tumour markers in monitoring epithelial ovarian cancer. Br J Cancer 2000;82:1535-8. [PubMed]
- Bast RC Jr, Xu FJ, Yu YH, et al. CA 125: the past and the future. Int J Biol Markers 1998;13:179-87. [PubMed]
- Menon U, Jacobs IJ. Ovarian cancer screening in the general population. Curr Opin Obstet Gynecol 2001;13:61-4. [Crossref] [PubMed]
- Verheijen RH, von Mensdorff-Pouilly S, van Kamp GJ, et al. CA 125: fundamental and clinical aspects. Semin Cancer Biol 1999;9:117-24. [Crossref] [PubMed]
- Rustin GJ, Nelstrop AE, Tuxen MK, et al. Defining progression of ovarian carcinoma during follow-up according to CA 125: a North Thames Ovary Group Study. Ann Oncol 1996;7:361-4. [Crossref] [PubMed]
- Rustin GJ, Marples M, Nelstrop AE, et al. Use of CA-125 to define progression of ovarian cancer in patients with persistently elevated levels. J Clin Oncol 2001;19:4054-7. [PubMed]
- Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem 2001;276:27371-5. [Crossref] [PubMed]
- Chu LC, Tsai HL, Wang H, et al. Posttreatment FDG PET/CT in predicting survival of patients with ovarian carcinoma. EJNMMI Res 2016;6:42. [Crossref] [PubMed]


