Peritoneal metastasis in relation to outcome and therapeutic strategy in gastric cancer
Introduction
Despite improvements in the surgical treatment of gastric adenocarcinoma, a high recurrence rate persists, with a 5-year overall survival rate for all diagnosed patients of only 24.5% in Europe (1) and 40–60% in Asia (2,3). In addition, the recurrence rate remains high particularly in patients with advanced stage disease. Among patients receiving R0 resection, 79% have documented recurrences within 2 years, and the median time to death from the time of recurrence is 6 months (4).
For resectable gastric cancer, most surgeons in China perform gastrectomy with D2 lymph node dissection. Despite curative section with extended nodal dissection, many patients, especially those with stage III disease, develop locoregional recurrence (LR), peritoneal recurrence (PR), or distant metastasis (5). A recent study of 1,178 patients with metastatic or recurrent gastric cancer showed that about 46% of patients had peritoneal metastases and about 30% had liver metastases (6). Several other clinical studies have reported recurrence patterns in a population of patients with early stage to advanced disease (4,7-10), showing that 30–54% of patients had PR alone or in combination. “Second-look” laparotomy is probably the best attempt to identify early LR and PR. However, routine second-look surgery has not proven worthwhile for gastric cancer because while it can allow for an earlier diagnosis, it does not improve recovery following treatment (11). Few studies have specifically analyzed patterns of metastasis in stage IV gastric cancer patients. In several clinical trials on advanced gastric cancer, peritoneal metastasis was observed in 29–38% of patients with other target lesions (12-15).
Understanding patterns of recurrence or metastasis can guide the selection of adjuvant treatment after curative resection or enhanced local treatment in stage IV patients. Therefore, in present study, we analyzed the recurrence or metastasis patterns in locally advanced and metastatic gastric cancer patients and identified potential risk factors. The relationship between patterns of recurrence or metastasis and overall survival was also investigated.
Methods
A total of 349 patients diagnosed with stage III or IV gastric cancer from 2006 to 2013 at Drum Tower Hospital were identified. The median age was 60.7 years (range, 34–83 years). Staging of the primary tumor was performed using the AJCC 7th criteria. Of the 349 patients, 52 (14.9%) were stage IIIA, 107 (30.6%) were stage IIIB, 55 (15.8%) were stage IIIC, and 135 (38.7%) were stage IV. All stage III patients underwent curative D2 gastrectomy after a multidisciplinary meeting. All patients received 5-fluorouracil and oxaliplatin based chemotherapy for least four cycles after pathological diagnosis. Patients who received neoadjuvant treatment were excluded in this study. All stage IV patients received 5-fluorouracil-based palliative chemotherapy after diagnosis. Patients with multiple primary cancer sites were excluded in this study. Patients were followed closely until July 2014; the median length of follow-up was 15 months (range, 2–86 months). Routine follow-up was conducted every 3 months during the first two years and every 6 months thereafter, for a total of 5 years and consisted of physical examination, laboratory test, chest radiography, abdominopelvic ultrasonography, and computed tomography.
Although some patients developed multiple recurrence or metastasis episodes, the main patterns of recurrence or metastasis were recorded as the first site of detectable failure at the time of disease progression for stage III patients or initial diagnosis for stage IV patients. Recurrences or metastasis were categorized into locoregional, peritoneal, or distant patterns. LR or locoregional metastasis included anastomotic recurrence, dominant masses in the gastric bed or upper abdominal retroperitoneal lymph nodes, such as the perigastric, retropancreatic, mesenteric, para-aortic, and hepatoduodenal nodes. PR or peritoneal metastasis was documented by positive cytology in ascitic fluid or by convincing peritoneal nodules on cross-sectional imaging. Krukenberg’s tumors were considered peritoneal. Distant sites included the supraclavicular lymph nodes and other solid organs, such as liver, lung, bone, and brain. The study was approved by institutional ethics board of Drum Tower Hospital (NO. 2014-019-02).
Statistical analysis
Potential predictive factors for a recurrence or metastasis pattern were analyzed with the χ2 and Fisher’s exact test for univariate comparisons. Survival was estimated by the Kaplan-Meier method, and the long-rank test was used to determine significance. Cox logistic regression was used for multivariate analysis. Statistical analysis was carried out with SPSS, version 17 (SPSS, Chicago, IL, USA). Two-sided P values <0.05 were considered statistically significant.
Results
Patterns of recurrence or metastasis
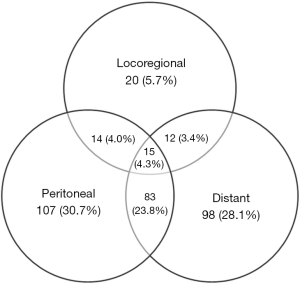
The main patterns of recurrence or metastasis in the 349 patients are demonstrated in Figure 1. Overall, 225 (64.5%) patients had only one recurrence or metastasis pattern, 109 (31.2%) had two involved areas, and 15 (4.3%) had involvement of all three areas. In patients with a single pattern, the most common site was peritoneal metastasis followed by distant and locoregional metastasis (30.7%, 28.1%, and 5.7%, respectively). In addition, when patients with multiple simultaneous patterns were included, these values increased to 62.8%, 59.6%, and 17.5%, respectively. The most common combined pattern was peritoneal and distant recurrence (DR) or metastasis, which was observed in 83 (23.8%) patients. PR or peritoneal metastasis was detected alone or in combination with other patterns in 219 patients (62.8%). Of the 349 patients, 283 (81.1%) had peritoneal or liver metastasis at the time of recurrence or diagnosis.
PR or peritoneal metastasis was detected as any part of the pattern in 131 patients (61.2%) with stage III and 88 patients (65.2%) with stage IV disease. Distant sites were involved as any part of recurrence or metastasis in 122 stage III patients (50.7%) and 86 stage IV patients (63.7%). The locoregional area was involved as any part of recurrence or metastasis in 45 stage III patients (21.0%) and 16 stage IV patients (11.9%).
Predicting the pattern of recurrence or metastasis
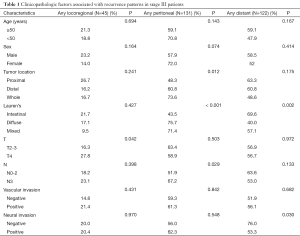
The association between clinicopathological factors and recurrence or metastasis patterns was analyzed separately for stage III and stage IV patients because detailed information of pathological classifications was unavailable for most stage IV patients. As shown in Table 1, stage III patients with T4 had a higher incidence of LR (P=0.042) as any of the recurrence pattern when compared with those of T2 and T3. Distal or whole stomach location (P=0.012), diffuse or mixed subtype (P<0.001), and advanced N stage (P=0.029) was associated with the presence of PR as any part of the recurrence pattern. Intestinal subtype (P=0.002) and negative vascular invasion (P=0.030) were associated with DR as any part of the recurrence pattern in stage III patients. No association was found between the metastasis pattern and patient characteristics such as age, sex, or tumor location in stage IV patients.
Full table
Survival and pattern of recurrence or metastasis
The median overall survival time was 14.0 months (95% CI: 12.4–15.6 months) for all 349 patients, 18.0 months (95% CI: 15.0–21.0 months) for stage III patients, and 9.0 months (95% CI: 7.7–10.3 months) for stage IV patients.
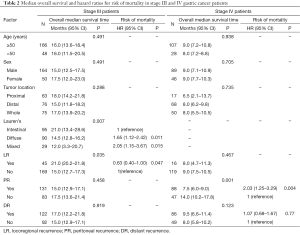
Among patients with stage III disease, the median survival was 14.5 months (95% CI: 12.8–16.2 months) for those with the diffuse subtype and 12.0 months (95% CI: 3.3–20.7 months) for those with the mixed subtype, compared to 21.0 months (95% CI: 13.4–28.6 months) for those with the intestinal subtype (P=0.007). The median survival was 21.0 months (95% CI: 20.2–21.8 months) for those with LR compared to 15.0 months (95% CI: 12.7–17.3 months) for those with PR or DR (P=0.035; Figure 2). Multivariate analyses demonstrated a higher risk of death in patients with the diffuse (HR: 1.646; 95% CI: 1.120–2.418; P=0.011) or mixed subtype (HR: 2.052; 95% CI: 1.147–3.672; P=0.015) when compared with patients of intestinal subtype. A lower risk of death was observed in patients with LR (HR: 0.628; 95% CI: 0.393–0.995; P=0.047; Table 2) comparing to those with PR or DR.

Full table
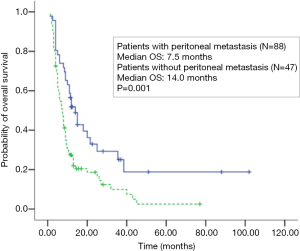
Among stage IV patients, shorter survival was observed in patients with peritoneal metastasis (7.5 months, 95% CI: 6.0–9.0 months) compared to those without peritoneal metastasis (14.0 months, 95% CI: 10.2–17.8 months; P=0.001; Figure 3). Multivariate analyses demonstrated that peritoneal metastasis was the only factor associated with a higher risk of death (HR: 2.026; 95% CI: 1.249–3.287; P=0.004).
When we calculated survival time after recurrence in stage III patients and performed survival analysis in all 349 patients together, the median survival duration was 7.0 months (95% CI: 5.7–8.3 months) for those with peritoneal metastasis compared to 10.0 months (95% CI: 7.6–12.4 months) for those without peritoneal metastasis (P=0.012). A higher risk of death was observed in patients with peritoneal metastasis (HR: 1.367; 95% CI: 0.996–1.877; P=0,050) compared to those without peritoneal metastasis after multivariate analyses.
Discussion
Patients with advanced stage disease show a poor prognosis, even after curative gastrectomy with extended lymph node dissection (16). Many investigators have analyzed recurrence patterns of gastric cancer patients after curative surgery, but the data have shown variable incidences of these patterns. Schwarz et al. (10) found that the most common pattern was distant metastasis while Eom et al. (17) found hematogenous metastasis to be most common among patients with early recurrence and LR and PR among patients with late recurrence using a cutoff time of 1 year after curative resection for patient subgroups. This disagreement was attributed to differences in patient cohorts undergoing evaluation, the cutoff at which recurrence was determined, and the methods for determining recurrence patterns. In addition, autopsy studies revealed only end-stage disease, but not early recurrence patterns, and re-operation series probably reflect early LR and PR. Routine second-look surgery does not improve patient prospects of recovery; therefore, clinical detection based on radiologic studies is the current clinical practice (8). Furthermore, clarifying the relationship between clinicopathological factors and patterns of recurrence may help to determine the best treatment or follow-up program.
In our present study, among stage III patients after D2 gastrectomy, very advanced T stage was associated with LR; distal or whole stomach location, diffuse or mixed subtype, and advanced N stage were associated with PR; and intestinal subtype and negative vascular invasion were associated with DR. When related to patient outcome, patients with PR or DR had shorter overall survival time than those with LR (15 vs. 21 months). Several studies have shown that postoperative chemoradiotherapy improves local recurrence in patients after gastrectomy; however, this improvement did not translate into a significant difference in 2-year overall survival (18). This may be explained by the results of the present study, in which we found the LR pattern was not an unfavorable predictive factor of survival in stage III gastric cancer patients. Radiotherapy is not currently included as a component of adjuvant therapy in most of the Asian cancer institutions because of the wide spread use of gastrectomy with extended lymph node dissection. However, understanding LR may allow individualized and more effective radiotherapy strategies. Chang et al. (5) identified the most commonly involved lymph modes of recurrence and the factors affecting survival in stage III (N3) gastric cancer patients who underwent D2 gastrectomy. Distinct patterns of regional recurrence in stage III gastric cancer patients can help in identifying potential candidates and determining lymph node target volume for postoperative radiotherapy.
PR appears to be the most common recurrence or metastasis patterns in stage III (61.2%) and stage IV (65.2%) patients. Among all patients, 81.1% developed metastasis in the peritoneal cavity, which includes peritoneal and liver metastasis. Peritoneal carcinosisis reported to be present in 5–20% of early gastric cancer patients considered for potentially curative resection (19,20). Peritoneal metastasis of gastric cancer is considered terminal with a median survival time of only 3.1 months (21). Despite recent improvement in chemotherapy regimens for recurrent cancer, the therapeutic effect of systemic chemotherapy on peritoneal carcinosis remains extremely limited. This may be due to the peritoneum–plasma barrier, which prevents effective drug delivery from the systemic circulation into the peritoneal cavity (22).
Considering the natural history of gastric cancer, the use of intraperitoneal chemotherapy (IPC) as a targeted adjuvant treatment may be effective as a prophylactic/therapeutic approach. IPC allows for a high intraperitoneal drug concentration to directly act on free-tumor-cells and peritoneal nodules. Drugs absorbed through the peritoneum enter the portal vein and exert chemotherapeutic effects on the liver (23). A recent meta-analysis including 20 prospective randomized controlled trials of 2,145 patients demonstrated that IPC improves survival and decreases the incidence of peritoneal failure or distant metastasis compared to surgery alone (24).
In advanced gastric cancer, cancer cells released from the primary site may cause intraperitoneal metastases (25). This potential was incorporated into the AJCC/UICC TNM classification (7th edition), which modified the recommendations to include routine peritoneal washing cytology in patients with locally advanced gastric cancer. Positive peritoneal cytology has previously been reported to be an independent predictor of prognosis in curatively resected patients with locally advanced gastric cancer (26) and in patients with suspected serosal invasion by gastric cancer (27). IPC should be considered as part of standard clinical practice for the treatment of advanced gastric cancer with or without overt peritoneal carcinosis. Several phase I and phase II clinical trials have been performed to evaluate the efficacy and tolerability of IPC in gastric cancer patients with peritoneal metastasis and/or cancer cells on peritoneal cytology (28,29). However, cautions should be taken in selecting patients for IPC according to histological differentiation and ascite baseline. Furthermore, the presence of amplified biomarker mRNA has been clinically correlated with worse outcome by peritoneal lavage evaluation with reverse transcription polymerase chain reaction (RT-PCR) in gastric cancer patients. RT-PCR for carcinoembryonic antigen (CEA) mRNA increases the detection of submicroscopic peritoneal disease and is more sensitive than conventional cytology (30).
In conclusion, the clinical detectable patterns of metastasis or recurrence varied according to certain characteristics of gastric cancer patients. However, it remains difficult to generate a predictive model for recurrence or metastasis patterns. Peritoneal metastasis was the most common pattern in our cohort and represented dismal outcome for gastric cancer. Proper selection of systemic (intravenous) and/or regional (intraperitoneal) chemotherapy may be a promising approach to improve the prognosis of advanced gastric cancer.
Acknowledgments
Funding: This work was funded by grants from the National Natural Science Foundation of China (Grant No. 81220108023, 81370064, 81572329) and the Fundamental Research Funds for the Central Universities (20620140729). The funding sources had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.01.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Drum Tower Hospital (NO. 2014-019-02). Informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sant M, Allemani C, Santaquilani M, et al. EUROCARE-4. Survival of cancer patients diagnosed in 1995-1999. Results and commentary. Eur J Cancer 2009;45:931-91. [Crossref] [PubMed]
- Zeng WJ, Hu WQ, Wang LW, et al. Long term follow up and retrospective study on 533 gastric cancer cases. BMC Surg 2014;14:29. [Crossref] [PubMed]
- Matsuda T, Saika K. The 5-year relative survival rate of stomach cancer in the USA, Europe and Japan. Jpn J Clin Oncol 2013;43:1157-8. [Crossref] [PubMed]
- D'Angelica M, Gonen M, Brennan MF, et al. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg 2004;240:808-16. [Crossref] [PubMed]
- Chang JS, Lim JS, Noh SH, et al. Patterns of regional recurrence after curative D2 resection for stage III (N3) gastric cancer: implications for postoperative radiotherapy. Radiother Oncol 2012;104:367-73. [Crossref] [PubMed]
- Jo JC, Ryu MH, Koo DH, et al. Serum CA 19-9 as a prognostic factor in patients with metastatic gastric cancer. Asia Pac J Clin Oncol 2013;9:324-30. [Crossref] [PubMed]
- Deng J, Liang H, Wang D, et al. Investigation of the recurrence patterns of gastric cancer following a curative resection. Surg Today 2011;41:210-5. [Crossref] [PubMed]
- Yoo CH, Noh SH, Shin DW, et al. Recurrence following curative resection for gastric carcinoma. Br J Surg 2000;87:236-42. [Crossref] [PubMed]
- Youn HG, An JY, Choi MG, et al. Recurrence after curative resection of early gastric cancer. Ann Surg Oncol 2010;17:448-54. [Crossref] [PubMed]
- Schwarz RE, Zagala-Nevarez K. Recurrence patterns after radical gastrectomy for gastric cancer: prognostic factors and implications for postoperative adjuvant therapy. Ann Surg Oncol 2002;9:394-400. [Crossref] [PubMed]
- Böhner H, Zimmer T, Hopfenmüller W, et al. Detection and prognosis of recurrent gastric cancer--is routine follow-up after gastrectomy worthwhile? Hepatogastroenterology 2000;47:1489-94. [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [Crossref] [PubMed]
- Boku N, Yamamoto S, Fukuda H, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol 2009;10:1063-9. [Crossref] [PubMed]
- Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 2008;9:215-21. [Crossref] [PubMed]
- Ahn HS, Lee HJ, Hahn S, et al. Evaluation of the seventh American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer 2010;116:5592-8. [Crossref] [PubMed]
- Eom BW, Yoon H, Ryu KW, et al. Predictors of timing and patterns of recurrence after curative resection for gastric cancer. Dig Surg 2010;27:481-6. [Crossref] [PubMed]
- Dikken JL, Jansen EP, Cats A, et al. Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol 2010;28:2430-6. [Crossref] [PubMed]
- Kuramoto M, Shimada S, Ikeshima S, et al. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg 2009;250:242-6. [Crossref] [PubMed]
- Ikeguchi M, Oka A, Tsujitani S, et al. Relationship between area of serosal invasion and intraperitoneal free cancer cells in patients with gastric cancer. Anticancer Res 1994;14:2131-4. [PubMed]
- Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 2000;88:358-63. [Crossref] [PubMed]
- Jacquet P, Sugarbaker PH. Peritoneal-plasma barrier. Cancer Treat Res 1996;82:53-63. [Crossref] [PubMed]
- Speyer JL, Sugarbaker PH, Collins JM, et al. Portal levels and hepatic clearance of 5-fluorouracil after intraperitoneal administration in humans. Cancer Res 1981;41:1916-22. [PubMed]
- Coccolini F, Cotte E, Glehen O, et al. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J Surg Oncol 2014;40:12-26. [Crossref] [PubMed]
- Yonemura Y, Kawamura T, Bandou E, et al. The natural history of free cancer cells in the peritoneal cavity. Recent Results Cancer Res 2007;169:11-23. [PubMed]
- Fukagawa T, Katai H, Saka M, et al. Significance of lavage cytology in advanced gastric cancer patients. World J Surg 2010;34:563-8. [Crossref] [PubMed]
- Lee SD, Ryu KW, Eom BW, et al. Prognostic significance of peritoneal washing cytology in patients with gastric cancer. Br J Surg 2012;99:397-403. [Crossref] [PubMed]
- Ishigami H, Kitayama J, Kaisaki S, et al. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol 2010;21:67-70. [Crossref] [PubMed]
- Kitayama J, Ishigami H, Yamaguchi H, et al. S-1 plus intravenous and intraperitoneal Paclitaxel for gastric cancer with peritoneal metastasis. Gastrointest Cancer Res 2012;5:S10-3. [PubMed]
- Wong J, Kelly KJ, Mittra A, et al. Rt-PCR increases detection of submicroscopic peritoneal metastases in gastric cancer and has prognostic significance. J Gastrointest Surg 2012;16:889-96; discussion 896. [Crossref] [PubMed]