
Aspirin inhibits the proliferation of canine mammary gland tumor cells in vitro and in vivo
Introduction
Canine mammary gland tumors (CMGTs) are the most frequently diagnosed tumors in intact female dogs, 50% of which are malignant (1). Currently, traditional surgery still is the main treatment for CMGT cases. However, approximately 50% of malignant cases expressing metastatic tumor will probably develop to recurrent cases (2,3). For malignant CMGTs, surgery combination with systemic chemotherapy is essential for promising prognosis. Exploring novel therapeutic agents to improve the survival rate of dogs with CMGTs is necessary and urgent.
Aspirin (acetylsalicylic acid), a non-steroidal anti-inflammatory drug (NSAID), was widely used to manage pain, arthritis, and cardiovascular diseases (4). Studies have shown that Aspirin inhibits the growth of various human malignant tumor cells in vitro and in vivo, including breast cancer (5,6), colorectal cancer (7,8), osteosarcoma (9) and prostate cancer (10). In recent years, emerging evidences indicate the beneficial effect of Aspirin in reducing the incidence of human cancer and even decreasing cancer mortality. For example, daily use of aspirin is associated with a significant reduction in the incidence of colorectal adenomas in patients with previous colorectal cancer (11). In human breast cancer, some studies have found that aspirin use is associated with a decreased risk of breast cancer (12). But there are inconsistent reports that regular Aspirin use did not reduce the risk of breast cancer and history of Aspirin did not appear to be protective or associated with improving clinical outcomes or survival rate among breast cancer patients (13,14). The beneficial impact of the use of Aspirin in humans with breast cancer has not yet been confirmed. Moreover, the role anti-tumor effect of Aspirin in CMGTs has not been explored thus far.
According to accumulated studies, Aspirin acts through decreasing the expression of COX-2 and inhibiting the growth of various cancers. High expression of COX-2 has been identified in many cancers including of CMGTs (15). COX-2 participates in different steps of the carcinogenetic process and also in tumor angiogenesis. However, some studies indicated that Aspirin has a COX-2-independent anti-tumor effect (6,7,16). Moreover, apoptosis and cell cycle arrest were considered to be the important factors where Aspirin inhibits the proliferation of cancer cells. For example, Aspirin could even induce apoptosis of cancer cells at dose of 2.5 mM in multiple myeloma (17). The remarkable effect on cell cycle arrest by Aspirin was usually detected at the dose of more than 5 mM in human colonic tumor cells (18).
Although the crucial role of Aspirin in the inhibition of cancer occurrence and growth has been recognized, the precise mechanism is largely unknown. In the present study, we tried to provide comprehensive evidence at the cellular level and in the animal models to investigate the anti-tumor effect of Aspirin and its mechanism of action on CMGTs. Our current research could lay a foundation for further clinical trials in the use of Aspirin in veterinary medicine. Due to mammary gland tumors sharing common features between dogs and humans, CMGTs are considered to be excellent models for human breast cancer. Therefore, advanced strategy findings in veterinary medicine are equally significant to human medicine.
Methods
Reagents and cell culture
Aspirin purchased from Sigma-Aldrich (St. Louis, MO, USA) was dissolved in dimethyl sulfoxide (DMSO) and kept at −20 °C for studies. Antibodies against cyclin D1 and E2F1 were purchased from Abcam Company (Cambridge, UK). Antibodies against CDK4, P21, PARP, Bcl-2 and BAX were purchased from Wanleibio (Shenyang, Liaoning, China). An antibody against β-actin was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
CHMm and CHMp cells were isolated from metastatic and primary lesions of a 12-year-old female dog. The two cell lines have been maintained for more than 60 generations without noticeable changes in their morphologies and proliferation characteristics (19). Cells were maintained in DMEM supplemented with 1% penicillin-streptomycin and 10% fetal bovine serum at 37 °C under a humidified atmosphere containing 5% carbon dioxide.
Cell viability assay
The cells were harvested and seeded in 96-well plates at a density of 3,000 cells per well. According to experiment design, cells were divided into untreated group and Aspirin-treated groups including three concentrations, 2.5, 5.0 and 10 mM. Then, the cells were incubated for 12, 24, 36, and 48 h at 37 °C, respectively. As described, the cell viability was measured by Cell Counting Kit-8 (CCK-8; Dojindo, Japan).
Clone formation assay
Cells were seeded in 6-well culture plates in triplicates at a density of 500 cells/well in 2 mL medium containing 10% FBS. After 24 hrs, cultures were replaced with fresh medium containing 5% FBS as control, or the same medium containing 2, 5 or 10 mM Aspirin. The plates were incubated at 37 °C with 5% CO2 in a humidified incubator for 10 days. Then the colonies were fixed with 4% paraformaldehyde, stained with 0.1% crystal violet solution for 15 min at room temperature, and washed with PBS, finally the colonies that containing more than 50 cells were counted.
Apoptosis flow-cytometry analysis
Aspirin-induced apoptosis was detected using an Annexin-V-FITC apoptosis assay kit (Beyotime, Jiangsu, China). Treated cells (0, 2.5, 5 and 10 mM Aspirin for 36 hours) were harvested; the assay was performed according to the manufacturer’s instruction. The data were analyzed using the CellQuest (Becton-Dickinson, San Jose, CA, USA) software.
Cell cycle flow-cytometry analysis
Control and Aspirin-treated cells were trypsinised and fixed overnight at –20 °C in 70% ice-cold ethanol. After fixation, the cells were washed twice with PBS and stained with 50 µg/mL propidium iodide (Solarbio, Beijing, China) and 0.1 mg/mL RNaseA (Solarbio, Beijing, China) for 30 min at 37 °C. Then the stained cells were immediately analyzed on Becton Dickinson FACS (Becton-Dickinson, San Jose, CA, USA) while the cell cycle distributions were analyzed using Modfit LT 4.0 software (Verity Software House, Topsham, USA).
Western blotting
The cells were briefly lysed in RIPA buffer (Beyotime, Jiangsu, China) solution containing protease inhibitor and phosphatase inhibitor cocktails (Roche). The protein extracts were quantified by BCA assay kit (Beyotime, Jiangsu, China). Equivalent amounts of protein (20–30 µg) samples were resolved using SDS-PAGE and transferred to polyvinylidene difluoride membranes for Western blotting. The membranes were blocked in 5% non-fat milk in TBST, and hybridized at 4 °C overnight with antibodies against PARP (1:500 dilution), Bcl-2 (1:500 dilution), BAX (1:500 dilution), Cyclin D1 (1:1,000 dilution), CDK4 (1:500 dilution), E2F1 (1:1,000 dilution), p21 (1:1,000 dilution) and beta-actin (1:1000 dilution), respectively. Then, the membranes were incubated for 1 h with Alexa Fluor 800-labeled goat anti-mouse IgG secondary antibody (Odyssey). Specific proteins were detected using an Odyssey infrared imaging system (LiCor BioSciences, Bad Homburg, Germany).
Tumor Xenograft
Six-week-old female Balb/c-nude mice were obtained from Slaccas (Slaccas Laboratory Animal, Shanghai, China). According to the National and Institutional Guidelines for Animal Care and Use. All experiment procedures were performed under protocols approved by the Northeast Agricultural University Ethics Committee for the use of laboratory animals. For CMGTs xenograft growth of orthotopic animal model, CHMm cells were injected subcutaneously into the right side of axillary of each mouse to initiate tumor growth. After 7 days, the tumors reached a mean diameter of 5 mm in all recipients. Then tumor-bearing mice were randomly divided into control and treated groups (n=6). The Aspirin-treated group was injected intraperitoneally at 100 mg/kg body weight per day for 3 weeks whilst the control group received the same amount of physiological saline only. Throughout the study, body weight and diet consumption were recorded every three/four days. After the initiation of the Aspirin administration, an external caliper was used to measure the size of the tumor and the tumor volume was calculated using the formula 1/2× (major axis) × (minor axis)2. At the end of the experiment, all the animals were sacrificed and the tumors were excised, weighed, then fixed in 4% paraformaldehyde and embedded in paraffin for further analysis.
Statistical Analysis
The data is presented as the mean ± SE. The error bars indicate the SE. Statistical comparisons between treated and control groups were calculated by Student’s t-tests using GraphPad Prism6, and multiple groups were determined by ANOVA test. A value of P<0.05 was considered to be significant; P<0.01 and P<0.001 was considered to be strongly significant.
Results
Aspirin inhibits CMGT cells Proliferation in vitro
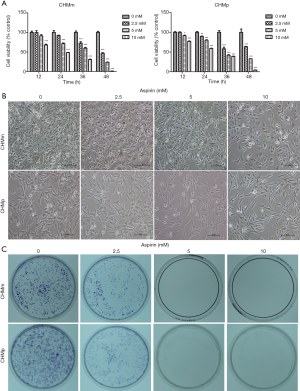
In order to determine whether Aspirin inhibits the proliferation of CMGT cells in vitro, we evaluated the effect of the drug on CHMm and CHMp cell lines using CCK-8 assay. Cell viability was measured 12, 24, 36, and 48 h after cells were treated with 2.5, 5, and 10 mM Aspirin. As shown in Figure 1A and Figure 1B, Aspirin decreased the viability of CHMm and CHMp cell in a time- and concentration-dependent manner. CHMm was significantly more sensitive than CHMp, with half maximal inhibitory concentrations (IC50) of 2.4 and 3.3 mM, respectively, from a 48 h exposure. These results demonstrate that Aspirin inhibits the proliferation of CMGT cells.
Aspirin suppress colony formation of CMGT cells in vitro
We examined the colony formation capacity of CMGT cells in the presence or absence of Aspirin for 2 weeks. As shown in Figure 1C, Aspirin significantly reduced the colony formation of CHMm and CHMp cells in a dose-dependent manner. Aspirin attenuated clonogenic survival of CMGT cells at concentrations as low as 2.5 mM. Even at the high concentration of Aspirin (5, 10 mM), the colony formation of CHMm and CHMp cells did not occur. These results suggest that Aspirin treatment could suppress the colony formation of CMGT cells.
Aspirin triggers metastatic CMGT cells apoptosis
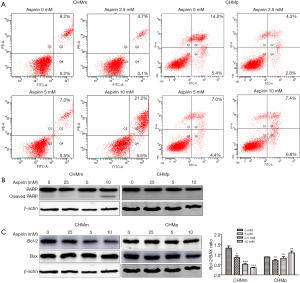
It is well known that Aspirin induces apoptosis in many cancers; this aim of the detection is investigate whether the proliferation inhibition of CGMT cells through Aspirin was related to the induction of cell apoptosis. CHMm and CHMp cells were treated with Aspirin (2.5, 5, 10 mM) for 36 h. As shown in Figure 2A, there were not significant differences of early apoptosis among 0, 2.5 5, 10 mM groups of CHMm cells. But the number of late apoptotic and necrosis cells of CHMm induced by Aspirin was significantly increased in group of 10 mM comparing with other groups. In addition, Western blot analysis showed that within the group of 10 mM, there was a significant increase in the cleaved-PARP protein level of CHMm cells (Figure 2B). In contrast, these effects were not observed in CHMp. Therefore, we deduced that only a high concentration of Aspirin could induce metastatic CGMT cells-apoptosis. Next, as indicated in Figure 2C, to ascertain whether Bcl-2 and Bax proteins were modulated by Aspirin in CMGT cells, a significant decline was observed in Bcl/Bax ratio compared to Aspirin treated CHMm cells and control. The induction of cell apoptosis by Aspirin might be related to the regulation of the Bcl-2/Bax system. However, the absence of similar results occurred in CHMp cells when compared with CHMm cells.
Aspirin induces cell cycle arrest in G0/G1 phases
In the investigation of whether Aspirin affected the cell cycle progression, CHMm and CHMp cells were treated with various concentrations of Aspirin for 36 h. As shown in Figure 3A, the results showed a greater increase in the G0/G1 phase proportion and a reduction in the percentage of cells in the S and G2/M phases.
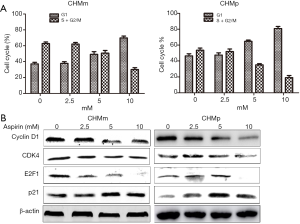
To further examine the expression of key proteins implicated in the transition of G0/G1 phase in CMGT cells by Aspirin. Apparently, western blot analysis showed that the cyclin D1, CDK4 and E2F1 protein levels decreased in Aspirin treated CHMm cells, while the cyclin-dependent kinase inhibitor (CDKIN) p21 increased. Similar results were also obtained in CHMp cells (Figure 3B). Taken together, these results demonstrate that Aspirin affects the expression of key proteins of the cell cycle and induces G0/G1 cell cycle arrest in CMGT cells.
Aspirin suppresses CMGT growth in xenograft mouse model
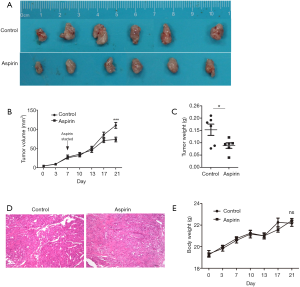
To investigate whether aspirin could inhibit the tumor growth in vivo, we established subcutaneous xenograft tumor models in nude mice. One week after implantation of CHMm cells, the mice were treated with or without Aspirin for 2 weeks. Compared with control mice, the tumor volumes in Aspirin-treated mice were significantly smaller (Figure 4A,B). Tumor mass weight difference in Aspirin-treated mice also supported the anti-tumor effects compared with that of the control mice (Figure 4C). Compared with control, the tumors from the mice of Aspirin-treated showed a higher level of fibrosis by hematoxylin and eosin (HE) staining of tumor sections (Figure 4D). Most importantly, Aspirin did not affect the animal weight and diet consumption (Figure 4E). All mice survived at the end of the experiment.
Discussion
Although previous studies has revealed the anti-tumor effect of Aspirin in many human cancer cells including breast cancer, the role of Aspirin in CMGT has not been explored thus far. In the present study, we demonstrate for the first time that Aspirin has not only effectively suppressed the proliferation and colony formation of CMGT cells in vitro, but has also inhibited the tumor growth in vivo. In vitro, Aspirin decreased the viability of tumor cell line in a concentration- and time-dependent manner. Aspirin selectively induce apoptosis of metastatic CGMT cells, and we investigated that Aspirin could inhibit cell cycle progression at the G0/G1 checkpoint leading to decreased viability of the cells. Collectively, our findings uncover the anti-tumor roles of Aspirin in CMGT cells.
Initially, we investigated whether Aspirin could cause the apoptosis of CMGT cells in a dose-dependent fashion. Previous studies revealed that apoptosis by Aspirin occurs in various human malignant tumor cells, including breast cancer (6), colon cancer (20), gastric cancer (21). In contrast, there were some challenging studies supporting that Aspirin marginally increased the frequency of apoptotic cancer cells when compared to controls (22). Our results demonstrated that noticeable apoptosis inducted by Aspirin occurred in CHMm cells incubated with 10 mM Aspirin. However, apoptosis could be not induced in CHMp by Aspirin. In present study, Aspirin decreased the ratio of Bcl-2 to Bax in CHMm cells, but failed to regulate the Bcl-2/Bax system in CHMp cells. The Bcl-2 family, including Bcl-2, Bax, Bad and Mcl-1, plays a pivotal role in the regulation of cell apoptosis (23). Previous studies revealed that Bcl-2 prevented apoptosis through blocking cytochrome c release from mitochondria (24). Accumulative researches also have suggested that overexpression of Bcl-2 could inhibit apoptosis via the formation of Bcl-2/Bax heterodimers, also the ratio of Bcl-2 to Bax appears to play a key role in a cell’s threshold for undergoing apoptosis (25,26). Moreover, some studies have shown that cancer cell death by Aspirin was mediated by apoptosis through the regulation of Bcl-2/Bax system (6,17). Based on these studies, we inferred that the difference effect of apoptosis by Aspirin between CHMp and CHMm should be related to the regulation of the Bcl-2/Bax system. These results indicate that the induction of apoptosis by Aspirin might be one of the major factors leading to the inhibition of proliferation of metastatic CGMT cells. In addition, the mechanism in which Aspirin selectively inducted apoptosis of metastatic CMGT cells has been defined but requires further investigation.
We further elucidate the molecular mechanisms of Aspirin suppressing CGMT. Although the effect of Aspirin leading to cell cycle arrest was previously demonstrated in human colon and breast cancers (7,18,27), currently little is known about this effect in CGMT cells. Cyclin D1 plays a critical role in the regulation of cell cycle at G1-S transition point and it binds to CDK4 and CDK6 to form active cyclin/CDK complexes that help to phosphorylate the retinoblastoma protein (Rb) (28). P21 is a CDKIN and is an important regulator of the cell cycle, which has been implicated in mechanisms of cell-cycle arrest (29). It also plays an important role in cell differentiation, senescence, apoptosis and tumorigenesis (30). Our results demonstrated that Aspirin could inhibit cell cycle progression in the G0/G1 phases with concomitant decreases in the expression of cyclin D1, CDK4, and E2F1. Aspirin increased the expression of p21 protein in CHMm and CHMp cells even at the concentration of 2.5 mM Aspirin treatment. It is worth noting that the dose of Aspirin (2.5 mM) did not induce apoptosis and cell cycle arrest on CMGT cells, but inhibited the proliferation of CMGT cells. We inferred that p21 might play a critical role at low-dose of Aspirin and deserve further investigation.
Previous reports have shown overexpression of the cyclin D1 gene (CCND1) has been found in several human tumors including breast cancers (31-33). Moreover, deregulated cyclin D1 expression also might play an important role in CMGT, also canine malignant mammary tumors exhibiting high cyclin D1 expression differ from cyclin D1-negative tumors in showing high proliferative rates (34). These studies indicate that inhibition of cyclin D1 seems to be an effective target for the treatment of CMGT using Aspirin.
There are an increasing number of studies reporting the anti-tumor effect of Aspirin because of its safety profile for a long-term use. In the present study, the dose of aspirin we used was 100 mg/kg daily each mice, which is markedly efficient to inhibit the tumor growth in immunodeficient xenograft mice. The concentration of 100 mg/kg daily in mice equals to 15 mg/kg daily in the dog, which was fit the middle dose (10–25 mg/kg) of Aspirin for analgesia according to clinical guidance. Our study indicates that Aspirin could exert its anti-cancer ability in vivo even at a safe dose level.
Platelets and platelet activation have been linked to key steps in cancer progression (35). The tumor cells covered with a coat of platelets could acquire the ability to evade the body’s immune system. Indeed, it has been shown that platelets protect tumors from TNF-a-mediated cytotoxicity (36). Aspirin is the most commonly used antiplatelet drug that inhibits platelet aggregation through cyclooxygenase-1 pathways. A recent study indicated that chemopreventive action of Aspirin against colon cancer cells may be dependent on blocking platelet activation and the ability of platelets to promote cancer cell growth and metastasis (37). In addition, low-dose Aspirin (100 mg per day) even could inhibit platelet aggregation and platelet thromboxane formation (38). These observations provided a hypothesis that Aspirin might act at low-doses through targeting platelet.
The pharmacodynamics (PD) and pharmacokinetics (PK) of Aspirin has been studied at low-doses in healthy volunteers. Inhibition of serum metabolite thromboxane B2 (TXB2) production was maximal about at 2 h postdose for Aspirin at a dose of 80 mg/day. Marked inhibition of TXB2 production and mean inhibition of urinary 11-dehydro-TXB2 excretion were both maintained through 24 h postdose with 80 mg Aspirin dose. Inhibition of arachidonic acid-induced platelet aggregation was sustained through 24 h postdose for single doses 80 mg Aspirin (at 24 h, 60.0% reduction). Peak plasma concentration (Cmax) for Aspirin at the doses of 80 mg was 504–900 ng/mL in plasma (39,40). Time to peak plasma concentration (Tmax) for Aspirin was attained 0.5–1.4 h postdose, and Aspirin concentrations were measurable up to 6 h postdose. But date on the PD and PK of Aspirin is incomplete in dog. This part of the data will be improved in the follow-up studies.
In summary, we herein provide comprehensive evidence for an inhibitory effect of aspirin on CMGT in vitro and vivo. The present work strongly suggests that Aspirin may be a chemotherapy drug for treating dog with CMGT. Our observations offered a basis for further clinical studies of Aspirin in veterinary medicine, which is equally significant to human medicine.
Acknowledgments
Funding: This study was supported entirely by the National Natural Science Foundation of China (Grant No. 31372492 and No. 31672617) and the funding of the Ministry of education of China for doctoral tutor (Grant No. 20122325110012).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.01.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Northeast Agricultural University Ethics Committee for the use of laboratory animals and conducted in accordance with the national and institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Moe L. Population-based incidence of mammary tumours in some dog breeds. J Reprod Fertil Suppl 2001;57:439-43. [PubMed]
- Philibert JC, Snyder PW, Glickman N, et al. Influence of host factors on survival in dogs with malignant mammary gland tumors. J Vet Intern Med 2003;17:102-6. [Crossref] [PubMed]
- Simon D, Schoenrock D, Baumgärtner W, et al. Postoperative adjuvant treatment of invasive malignant mammary gland tumors in dogs with doxorubicin and docetaxel. J Vet Intern Med 2006;20:1184-90. [PubMed]
- Sneader W. The discovery of aspirin: a reappraisal. BMJ 2000;321:1591-4. [Crossref] [PubMed]
- Sotiriou C, Lacroix M, Lagneaux L, et al. The aspirin metabolite salicylate inhibits breast cancer cells growth and their synthesis of the osteolytic cytokines interleukins-6 and -11. Anticancer Res 1999;19:2997-3006. [PubMed]
- Maity G, De A, Das A, et al. Aspirin blocks growth of breast tumor cells and tumor-initiating cells and induces reprogramming factors of mesenchymal to epithelial transition. Lab Invest 2015;95:702-17. [Crossref] [PubMed]
- Goel A, Chang DK, Ricciardiello L, et al. A novel mechanism for aspirin-mediated growth inhibition of human colon cancer cells. Clin Cancer Res 2003;9:383-90. [PubMed]
- Din FV, Valanciute A, Houde VP, et al. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology 2012;142:1504-15.e3. [Crossref] [PubMed]
- Liao D, Zhong L, Duan T, et al. Aspirin Suppresses the Growth and Metastasis of Osteosarcoma through the NF-κB Pathway. Clin Cancer Res 2015;21:5349-59. [Crossref] [PubMed]
- Lloyd FP Jr, Slivova V, Valachovicova T, et al. Aspirin inhibits highly invasive prostate cancer cells. Int J Oncol 2003;23:1277-83. [PubMed]
- Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med 2003;348:883-90. [Crossref] [PubMed]
- García Rodríguez LA, González-Pérez A. Risk of breast cancer among users of aspirin and other anti-inflammatory drugs. Br J Cancer 2004;91:525-9. [Crossref] [PubMed]
- Egan KM, Stampfer MJ, Giovannucci E, et al. Prospective study of regular aspirin use and the risk of breast cancer. J Natl Cancer Inst 1996;88:988-93. [Crossref] [PubMed]
- Li Y, Brasky TM, Nie J, et al. Use of nonsteroidal anti-inflammatory drugs and survival following breast cancer diagnosis. Cancer Epidemiol Biomarkers Prev 2012;21:239-42. [Crossref] [PubMed]
- Millanta F, Citi S, Della Santa D, et al. COX-2 expression in canine and feline invasive mammary carcinomas: correlation with clinicopathological features and prognostic molecular markers. Breast Cancer Res Treat 2006;98:115-20. [Crossref] [PubMed]
- Grösch S, Tegeder I, Niederberger E, et al. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J 2001;15:2742-4. [PubMed]
- Ding JH, Yuan LY, Huang RB, et al. Aspirin inhibits proliferation and induces apoptosis of multiple myeloma cells through regulation of Bcl-2 and Bax and suppression of VEGF. Eur J Haematol 2014;93:329-39. [Crossref] [PubMed]
- Subbegowda R, Frommel TO. Aspirin toxicity for human colonic tumor cells results from necrosis and is accompanied by cell cycle arrest. Cancer Res 1998;58:2772-6. [PubMed]
- Uyama R, Nakagawa T, Hong SH, et al. Establishment of four pairs of canine mammary tumour cell lines derived from primary and metastatic origin and their E-cadherin expression. Vet Comp Oncol 2006;4:104-13. [Crossref] [PubMed]
- Qiao L, Hanif R, Sphicas E, et al. Effect of aspirin on induction of apoptosis in HT-29 human colon adenocarcinoma cells. Biochem Pharmacol 1998;55:53-64. [Crossref] [PubMed]
- Wong BC, Zhu GH, Lam SK. Aspirin induced apoptosis in gastric cancer cells. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie 1999;53:315-318.
- Talarico G, Orecchioni S, Dallaglio K, et al. Aspirin and atenolol enhance metformin activity against breast cancer by targeting both neoplastic and microenvironment cells. Sci Rep 2016;6:18673. [Crossref] [PubMed]
- Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol 1998;16:395-419. [Crossref] [PubMed]
- Yang J, Liu X, Bhalla K, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 1997;275:1129-32. [Crossref] [PubMed]
- Korsmeyer SJ, Shutter JR, Veis DJ, et al. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol 1993;4:327-32. [PubMed]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol 2007;35:495-516. [Crossref] [PubMed]
- Raza H, John A, Benedict S. Acetylsalicylic acid-induced oxidative stress, cell cycle arrest, apoptosis and mitochondrial dysfunction in human hepatoma HepG2 cells. Eur J Pharmacol 2011;668:15-24. [Crossref] [PubMed]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 1999;13:1501-12. [Crossref] [PubMed]
- Klopfleisch R, Gruber AD. Differential expression of cell cycle regulators p21, p27 and p53 in metastasizing canine mammary adenocarcinomas versus normal mammary glands. Res Vet Sci 2009;87:91-6. [Crossref] [PubMed]
- Han Z, Wei W, Dunaway S, et al. Role of p21 in apoptosis and senescence of human colon cancer cells treated with camptothecin. J Biol Chem 2002;277:17154-60. [Crossref] [PubMed]
- Drobnjak M, Osman I, Scher HI, et al. Overexpression of cyclin D1 is associated with metastatic prostate cancer to bone. Clin Cancer Res 2000;6:1891-5. [PubMed]
- Betticher DC, Heighway J, Hasleton PS, et al. Prognostic significance of CCND1 (cyclin D1) overexpression in primary resected non-small-cell lung cancer. Br J Cancer 1996;73:294-300. [Crossref] [PubMed]
- Gillett C, Fantl V, Smith R, et al. Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res 1994;54:1812-7. [PubMed]
- Sfacteria A, Bertani C, Costantino G, et al. Cyclin D1 expression in pre-cancerous and cancerous lesions of the canine mammary gland. J Comp Pathol 2003;128:245-51. [Crossref] [PubMed]
- Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost 2011;9:237-49. [Crossref] [PubMed]
- Jurasz P, Alonso-Escolano D, Radomski MW. Platelet--cancer interactions: mechanisms and pharmacology of tumour cell-induced platelet aggregation. Br J Pharmacol 2004;143:819-26. [Crossref] [PubMed]
- Guillem-Llobat P, Dovizio M, Bruno A, et al. Aspirin prevents colorectal cancer metastasis in mice by splitting the crosstalk between platelets and tumor cells. Oncotarget 2016;7:32462-77. [PubMed]
- Lorenz RL, Schacky CV, Weber M, et al. Improved aortocoronary bypass patency by low-dose aspirin (100 mg daily). Effects on platelet aggregation and thromboxane formation. Lancet 1984;1:1261-4. [Crossref] [PubMed]
- Benedek IH, Joshi AS, Pieniaszek HJ, et al. Variability in the pharmacokinetics and pharmacodynamics of low dose aspirin in healthy male volunteers. J Clin Pharmacol 1995;35:1181-6. [Crossref] [PubMed]
- Patrick J, Dillaha L, Armas D, et al. A randomized trial to assess the pharmacodynamics and pharmacokinetics of a single dose of an extended-release aspirin formulation. Postgrad Med 2015;127:573-80. [Crossref] [PubMed]


