
Immunosuppressive role of γδ T cells in cancer: the other side of the coin
Development of cancer is associated with evolution of tumor microenvironment which subverts the immune response for its benefit. The tumor environment promotes the production of proinflammatory cytokines, leading to the accumulation of suppressive cells that inhibit antitumor immunity. In contrast, strong lymphocyte infiltration has been reported to be associated with an antitumor response and improved clinical outcome (1). The prevalence of suppressive or anti-tumor immune cells in the tumor environment determines the course of the disease. T cells expressing γδ T cell receptor (TCR) are one of the vital players in tumor infiltrating lymphocytes (TILs). The functional significance of γδ T cells in the tumor environment is still an enigma. The disparate properties of γδ T cells to recognise antigens in MHC unrestricted manner bestow an advantage to γδ T cells over αβT cells for anti-tumor immunity. γδ T cells specifically respond to the stress-induced MHC class I-related molecules MICA, MICB, and the UL16-binding proteins (ULBP) that are upregulated on malignant or stressed cells (2).
The activation of γδ T cells induces expression of perforins and granzymes, engagement of death-inducing receptors, such as FAS and expression of TNF-related apoptosis-inducing ligand (TRAIL) (3,4). IFNγ secreted by activated γδ T cells enhances MHC class I expression by tumour cells, thus promoting CD8+ T cell responses (4). The major subsets of γδ T cells, Vδ1+ (predominant in tissues) and Vδ2+ (predominant in the circulation) show cytolytic activity against solid and hematologic tumors (5). Meta-analysis of clinical trials using γδ T cells in patients with acute myeloid leukemia, chronic lymphocytic leukemia, non-small cell lung carcinoma, renal cell carcinoma, multiple myeloma, cervical cancer, etc. highlights the significance of γδ T cells as potential candidate for cell based immunotherapy in cancer patients (5). This suggests that it is possible to harness the antitumor properties of γδ T cells by in vitro and/or in vivo manipulation of γδ T cells. However, the functional properties of γδ T cells during progression of cancer in pre-invasive and clinically non apparent disease are not well understood.
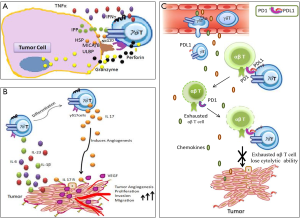
The emerging evidences in recent past have highlighted the tumor promoting functions of γδ T cells in patients. A common mediator of such functions is shown to be the cytokine IL17 (6). Using a methylcholanthrene (MCA)-induced fibrosarcoma model, it was first shown that γδ T cells were the main source of IL17 and in the absence of IL17, reduction in tumor growth and blood vessel density were observed (7). Similarly, in lung metastasis model, Tγδ17 cells were associated with detrimental effects observed in the tumor bearing host (8). Tγδ17 cells suppress CD8+ cytotoxic T lymphocytes by expansion and polarization of neutrophils in the mice bearing mammary tumours (9). It was reported that Tγδ17 cells induce mobilization of pro-tumor small peritoneal macrophages (SPM) as well as myeloid-derived suppressor cells (MDSCs) to the tumor bed which induce angiogenesis and immune suppression respectively (10,11). In human colorectal cancer Tγδ17 cells are reported to recruit MDSCs to the tumor bed and associate with clinical stage of patients (12). Recently our group has shown that Tγδ17 cells were elevated in tumor tissue and peripheral blood of gallbladder cancer patients and were associated with poor survival by inducing angiogenesis in the gallbladder tumor cells (13). Human Vδ1+γδ T cells were also reported to suppress CD4+ and CD8+ T cells and also impaired the maturation and T-cell priming capacity of dendritic cells (14). However, the molecular mechanism behind the immunosuppressive behaviour of γδ T cells is poorly understood.
Recent data from Daley and collaborators have provided compelling evidence to show the robust immunosuppressive effect of γδ T cells restraining antitumor αβT cells to support pancreatic ductal adenocarcinoma (PDA) (15). Previous reports show that in a murine model of pancreatic intraepithelial neoplasia (PanIN), mutation in KrasG12D induced infiltration of IL17 producing T cells to the tumor bed. The propensity of IL17 production by γδ T cells was shown to be higher than CD4+ T cells (16). However, the contribution of γδ T cells in pancreatic cancer progression remains elusive. Daley et al. addressed the functional role of γδ T cells in pancreatic cancer using invasive [C57BL/6-Trdctm1Mal mice harbouring orthotopically implanted Pdx1Cre;KrasG12D;Tp53R172H (KPC)-derived invasive PDA] and pre-invasive [p48Cre;KrasG12D (KC) mice harbouring pre-invasive tumor] murine PDA. It was shown that γδ T cells abundantly infiltrated the invasive PDA tissue and predominantly expressed Vγ4 than Vγ1. The PDA infiltrating γδ T cells showed upregulated expression of molecules associated with immunosuppression such as FAS ligand, NK1.1, CD39, JAML, and OX40. Moreover, these γδ T cells also expressed elevated levels of IL10 and FoxP3 than their splenic counterpart. In addition, the PDA infiltrating γδ T cells also expressed IL17, TNFα, IFNγ, NKG2D receptor, TLR4, TLR7 and TLR9 at higher level compared to γδ T cells in the spleen. This suggests that the γδ T cells were distinctly activated in PDA. Similar observations were obtained in 6-month-old KC mice, a model of pre-invasive PDA. In human PDA, γδ T cells comprise up to 75% of tumor infiltrating CD3+ T cells and were significantly increased than CD8+ T cells. Similar to murine models of PDA, Vγ9+ γδ T cells were absent in human PDA and predominantly belonged to CD45RA−CD27−T(effector memory) phenotype.
Daley et al. reported that PDA infiltrating γδ T cells showed elevated levels of CCR2, CCR5, and CCR6. The involvement of these chemokine receptors in recruitment of γδ T cells to the tumor bed was demonstrated using CCR2−/−, CCL2−/−, CCR5−/− and CCR6−/− mice with orthotopic KPC-derived tumor. Deletion of CCR2, CCL2, or CCR6 significantly reduced γδ T cell infiltration to the tumor bed which was correlated with reduced tumor burden.
The PDA infiltrating γδ T cells have significant role in pancreatic oncogenesis. The genetic deletion of γδ T cells (wild type KC mice crossed with Tcrδ−/− mice and challenged orthotopically with PDA) were associated with reduced dysplastic ducts and slower PanIN progression. Moreover, depletion of Vγ4+ γδ T cells in KC mice or in orthotopic KPC model (using neutralizing monoclonal antibody UC3-10A6) protected against tumor growth and showed increased survival. Notably, absence of γδ T cells did not affect pancreatitis suggesting the tumor promoting effect of γδ T cells was specific to PDA. However, γδ T cells failed to enhance proliferation or deregulate expression of oncogenic or tumor suppressor genes in transformed epithelial cells suggesting indirect involvement of γδ T cells on PDA progression.
Daley et al. observed that depletion of γδ T cells resulted in pronounced infiltration of activated CD4+ and CD8+ T cells. The infiltrated αβT cells expressed higher levels of CD44, ICOS, CTLA-4, granzyme B, OX40 and PD-1 in Tcrδ−/− mice. Further, the co-culture studies of γδ T cells with αβT cells showed contact dependent inhibition of activation of CD4+ and CD8+ T cells highlighting immunosuppressive behaviour of γδ T cells. Signalling through PD-1/PD-L1 induces T cell exhaustion (17). Daley et al. have shown that PDA infiltrating γδ T cells expressed elevated levels of PD-L1 and galactin-9 but decreased levels of other activating ligands including B7-2, ICOSL, and OX40L in orthotopic KPC and KC tumors. Blockade of PD-L1 in co-culture experiment of γδ T cells with αβT cells reversed the γδ T cells mediated suppression of CD4+ and CD8+ T cells. Moreover, blockade of PD-L1 and galectin-9 using neutralizing monoclonal antibodies (mAbs) in wild type mice protected from tumor progression but were ineffective at further inducing tumor protection in KC;Tcrδ−/− animals. Interestingly, it was observed that expression of PD-L1 on MDSCs was not affected and exhibited PD-1/PD-L1 dependent T cell inhibition in KC;Tcrδ−/− mice. However, MDSCs did not promote tumor growth in KC;Tcrδ−/− mice treated with checkpoint inhibitors. Daley et al. showed that αβT cells were in proximity to γδ T cells in the PDA. Myeloid cells were separated by great distances from αβT cells, in invasive murine PDA, pre-invasive disease and in human PDA. This suggests enhanced opportunity for γδ T cells to restrain the αβT cell activation in PDA compared to myeloid cells.
In summary, the present article by Daley et al. has provided the molecular mechanism of immunosuppressive behaviour of γδ T cells through PD-1/PD-L1 signalling imposing immune exhaustion of antitumor αβT cells. This study has revealed the suppressive behaviour of γδ T cells which adds new dimensions to the previously reported pro-angiogenic functions of γδ T cells in treatment naive condition (Figure 1). The monoclonal antibodies targeting PD-1/PD-L1 are in clinical trials in patients with melanoma, renal cancer, non-small cell lung, bladder and head and neck cancers (18). The present study has provided rationale for therapeutic use of anti-PD-1/PD-L1 antibodies in treatment of patients with PDA. Thus, for achieving therapeutic benefits of γδ T cells in clinical setting it is necessary to fine tune γδ T cells using appropriate blocking antibodies for IL17/IL17R and PD-1/PD-L1 pathway in combination with phosphoantigens and TLR agonists.
Acknowledgments
We acknowledge Department of Atomic Energy, Government of India for providing research fellowship to R Patil and SK Sureshbabu.
Funding: We thank Department of Biotechnology, Government of India, for Centre of Excellence grant (BT/01/CEIB/09/V/06) for our project cited as reference number 13.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Hao Feng (Experimental Surgical Research, Department of General, Visceral, Transplant, Vascular and Thoracic Surgery, Hospital of the LMU Munich, Munich, Germany).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.01.29). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Angell H, Galon J. From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol 2013;25:261-7. [Crossref] [PubMed]
- Born WK, Kemal Aydintug M, O'Brien RL. Diversity of γδ T-cell antigens. Cell Mol Immunol 2013;10:13-20. [Crossref] [PubMed]
- Dhar S, Chiplunkar SV. Lysis of aminobisphosphonate-sensitized MCF-7 breast tumor cells by Vγ9Vδ2 T cells. Cancer Immun 2010;10:10. [PubMed]
- Silva-Santos B, Serre K, Norell H. γδ T cells in cancer. Nat Rev Immunol 2015;15:683-91. [Crossref] [PubMed]
- Fisher JP, Heuijerjans J, Yan M, et al. γδ T cells for cancer immunotherapy: A systematic review of clinical trials. Oncoimmunology 2014;3:e27572 [Crossref] [PubMed]
- Patil RS, Bhat SA, Dar AA, et al. The Jekyll and Hyde story of IL17-Producing γδT Cells. Front Immunol 2015;6:37. [Crossref] [PubMed]
- Wakita D, Sumida K, Iwakura Y, et al. Tumor-infiltrating IL-17-producing gammadelta T cells support the progression of tumor by promoting angiogenesis. Eur J Immunol 2010;40:1927-37. [Crossref] [PubMed]
- Carmi Y, Rinott G, Dotan S, et al. Microenvironment-derived IL-1 and IL-17 interact in the control of lung metastasis. J Immunol 2011;186:3462-71. [Crossref] [PubMed]
- Coffelt SB, Kersten K, Doornebal CW, et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015;522:345-8. [Crossref] [PubMed]
- Rei M, Gonçalves-Sousa N, Lança T, et al. Murine CD27(-) Vγ6(+) γδ T cells producing IL-17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. Proc Natl Acad Sci U S A 2014;111:E3562-70. [Crossref] [PubMed]
- Ma S, Cheng Q, Cai Y, et al. IL-17A produced by γδ T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res 2014;74:1969-82. [Crossref] [PubMed]
- Wu P, Wu D, Ni C, et al. γδT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity 2014;40:785-800. [Crossref] [PubMed]
- Sudam Patil R, Umesh Shah S, Vinayak Shrikhande S, et al. IL17 producing γδT cells induce angiogenesis and are associated with poor survival in gallbladder cancer patients. Int J Cancer 2016;139:869-81. [Crossref] [PubMed]
- Peng G, Wang HY, Peng W, et al. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity 2007;27:334-48. [Crossref] [PubMed]
- Daley D, Zambirinis CP, Seifert L, et al. γδ T Cells Support Pancreatic Oncogenesis by Restraining αβ T Cell Activation. Cell 2016;166:1485-1499.e15. [Crossref] [PubMed]
- McAllister F, Bailey JM, Alsina J, et al. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell 2014;25:621-37. [Crossref] [PubMed]
- Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 2015;15:486-99. [Crossref] [PubMed]
- Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol 2015;27:39-46. [Crossref] [PubMed]


