
Effectiveness of cervical cancer therapy using neoadjuvant chemotherapy in combination with radical surgery: a meta-analysis
Introduction
Cervical cancer represents a common gynecologic malignancy in developed and developing countries (1). Approximately 500,000 new cases of cervical cancer are diagnosed each year with 70% of them diagnosed at an advanced stage (2). Both the size of the lesion and depth of stromal invasion are reported to have an important effect on survival, and thus the lesion diameter becomes the most important prognostic factor during cervical cancer treatment (3,4). According to the staging of cervical cancer by the International Federation of Gynecology and Obstetrics (FIGO) in 2009, cancers at stages IB-IIA (tumor size more than 4 cm) and FIGO stage IIB and above, are characterized by bulky tumor volume, deep cervical stromal invasion, and a high rate of lymph node metastasis (35–80%), having a high rate of recurrence after conventional radical surgery (RS) alone (5). These patients have a very poor prognosis with an overall 5-year survival rate of around 40% after conventional treatments. These facts have posed great challenge to the treatment of cervical cancer (6).
Recently, neoadjuvant chemotherapy (NACT) prior to surgery or radiotherapy has been proposed as a new therapeutic option for bulky or locally advanced cancers (6). NACT has been reported to be able to improve the pelvic control and eradicate the micrometastasis for advanced cervical tumor, and be regarded as an effective method in tumor volume reducing, which could greatly facilitate the surgical removal (7).
So far, numerous studies have been conducted to evaluate the effectiveness of combined treatment using both NACT and RS (8-24). However, the results of these studies are so contradictory. Specifically, Eddy et al. suggested that the combination of NACT and RS provided no additional objective benefit for cervical cancer patients than using the RS alone, and this conclusion was further validated by other studies (11,25,26). In contrast, other studies indicated that the combined treatment could improve the long-term disease-free survival (DFS) and overall survival (OS) (17,27). Therefore, no conclusion has been drawn on whether NACT combined with operational therapy, can improve the recurrence rate of cervical cancer. With the rapid accumulation of studies in these years, we performed a meta-analysis in this study, which involved as many reports as possible, to investigate the improvement of a combination of NACT and RS than RS alone for cervical cancer patients.
Methods
Identification of eligible publication and acquisition
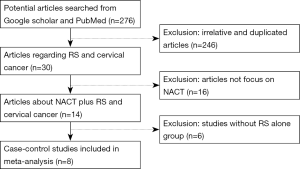
PubMed and Google Scholar database were searched with the following medical subject terms: “cervical cancer/carcinoma/tumor/neoplasm”, “NACT” and “RS”. The search included literature published from Jan. 2000 to Sep. 2016. Totally 276 results (108 from PubMed and 168 from Google Scholar) were acquired initially. Among these results, 246 literatures of irrelative or duplicated articles were excluded, resulting in 30 articles regarding RS and cervical cancer; Then 16 articles were eliminated due to irrelevance to NACT. Six articles without RS alone were further deleted. Therefore eight studies focusing on NACT plus RS and cervical cancer met with our requirement and were acquired altogether. Articles not focusing on NACT, RS and/or cervical cancer were excluded. Finally eight studies were employed in our meta-analysis. For each literature, the following data were extracted: the first author’s last name, year of publication, country, ethnicity, number of patients, ANCT and surgery performed, adjuvant therapy, DFS and OS rates (for both 2- and 5-year), and information on surgical-pathologic risk factors. The procedure of article collection was shown in Figure 1.
Statistical methods
STATA software (version 12.0) was used to analyze the collected data in this study. The improvement between case group and control group was evaluated using all databases by pooled odds ratios (ORs) with 95% confidence intervals (CIs). The heterogeneity assumption was assessed by I2 index. I2≤25% was assumed no significant heterogeneity between pooled data, while I2>75% was regarded as significant heterogeneous. We used Mantel-Haenszel (M-H) fixed-effect model for calculations unless there was a significant heterogeneity, in which DerSimonian and Laird (D-L) random-effected model was applied instead. ORs were calculated with each model within 95% confidence intervals. Forest plots were generated to visualize the results. Begg’s funnel plots and Egger’s regression asymmetry test was employed to evaluate potential publication bias. All P values were two-sided, and P<0.05 was considered statistically significant.
Results
Characteristic of data collected
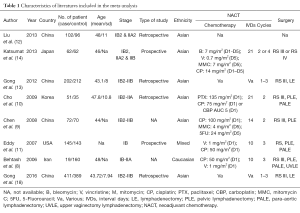
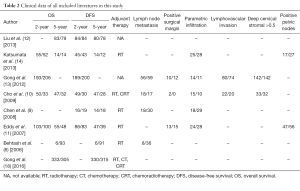
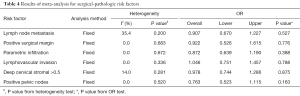
In our study, eight eligible case-control studies were collected, and characteristics of these studies were shown in Table 1. These studies involved 654 cases and 998 controls in total, whose ages are usually around 45 excluding study from Chen et al. (9) without relevant data. All data were recorded at late stage with bulk cancer volume (IB-IIA or above). Among all the studies, five focused on Asian population, while other two involved with Caucasian people. NACT was different among most studies except two studies (8,11), which performed the same NACT (intravenous vincristine 1 mg/m2 and cisplatin 50 mg/m2 every 10 days for three cycles). Surgery was performed according to RS III with different lymphadenectomy in seven studies and to RS III or IV in one study (14). Clinical data of all these studies were shown in Table 2. The overall and DFS rates were analyzed for 2 and 5 years separately. Survival data which are not available in some studies were shown as blank. In total, four studies possess 2-year OS data, of which all comes from Asian people; and six studies have 5-year OS data. Meanwhile, DFS were available in seven of the total studies. The subgroup meta-analyses by adjuvant therapy (RT, CT or CRT) are 3, 5, 4, and 6 studies for 2-year OS, 5-year OS, 2-year DFS, and 5-year DFS, respectively. To acquire complete understanding about the clinical efficacy of the treatment, we also included in this study with six surgical-pathologic risk factors including lymph node metastasis, positive surgical margin, parametric infiltration, lymphovascular invasion, cervical stromal depth and positive pelvic nodes.
Full table
Full table
Meta-analysis on comparison of NACT combined with RS vs. RS alone treatment
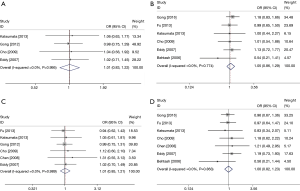
In order to assess the combination effect of NACT and RS treatment, we performed systemic survival analysis on available clinical data. The results of the overall and DFS rates between case (NACT combined with RS treatment) and control group (RS treatment alone) were shown in Table 3, and forest plot for each survival rate was shown in Figure 2. For all survival rates, I2 indexes were consistently equal to 0% with no statistically significant heterogeneity (P>0.05). In this regard, we used fixed-effect model for the calculation for all of them. Overall, we found no significant survival rate improvement for the combination of NACT and RS over the RS alone group (2-year OS: OR =1.008; 95% CI, 0.832–1.220; P=0.937; Figure 2A; 5-year OS: OR =1.054; 95% CI, 0.860–1.292; P=0.913; Figure 2B; 2-year DFS: OR =1.015; 95% CI, 0.853–1.207; P=0.870; Figure 2C; 5-year DFS: OR =1.001; 95% CI, 0.816–1.228; P=0.992; Figure 2D). Besides, we also found that there was no significant publication bias based on funnel plot for 2- and 5-year survival rates (as were shown in Figure 3). This was further confirmed by results of both Egger’s and Begg’s test, with P value larger than 0.05 in all cases, which was shown in Table 3. Figure 4 shows subgroup meta-analyses of the efficacy of NACT and FS on cervical cancer therapy according to adjuvant therapy. There was also no significant efficacy on OS or FDS in subgroup meta-analyses by adjuvant therapy. These results indicated that the cervical cancer patients with the additive treatment of NACT may have not any beneficial effect on survival rate than RS treatment alone.

Full table

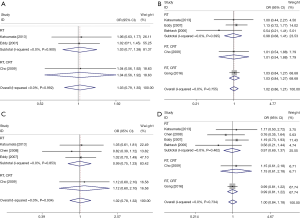
Moreover, to broaden the clinical implication of this comparison, we performed surgical-pathologic risk factors analysis, and results were shown in Table 4. Due to the insignificant statistics of the heterogeneity of I2 indexes, fixed model was used in this analysis. The lack of significant heterogeneity (P>0.05) found in all analysis, indicated the reliability of the results with no significant publication bias. Pooled analysis showed all p values were more than 0.05 for OR tests, suggesting that compared with control group, case group demonstrated little statistically significant improvement regarding each individual risk factor, including lymph node metastasis, positive surgical margin, parametric infiltration, lymphovascular invasion, cervical stromal depth and positive pelvic nodes. All these analyses further confirmed the previous conclusion, and demonstrated that the combination of NACT and RS combination therapy could have no improvement on RS treatment alone for cervical cancer therapy.
Full table
Discussion
In this study, we presented a meta-analysis to investigate the improvement of the combination of NACT plus RS over RS alone in the treatment of cervical cancer. This systematic review and meta-analysis of clinical trial showed that patients with additive NACT treatment may have no additional benefit for the long-term OS and DFS than using RS alone. Subgroup meta-analysis by adjuvant therapy and further assessments on six surgical-pathologic risk factors also confirmed this conclusion.
The rational that some researchers believe why NACT can enhance the clinical treatment of cervical cancer is apparent. The stage of the cervical cancer strongly decides the treatment of this cancer, of which the tumor size and volume have both been regarded as important prognostic factors for early cervical cancer. However, majority of cervical cancer patients have already reached late or advanced stage at diagnosis, which cannot be effectively eradicated by traditional radical surgeries (28). The potential advantages of NACT was thought to lies in its effectiveness in minimize tumor size, decreasing the number of micro metastases (29) and widening uninfiltrated resection area (6). Another advantage of NACT followed by surgery relies on the removal of potential chemo-resistant foci (30) diminishing the need for adjuvant treatment (31). Therefore it has been widely suggested that preoperative NACT could reinforce the efficacy of treatment for patients who do not meet the criteria for surgery (28,32).
However, in compliance with our study, objections to the usage of preoperative neoadjuvant therapy still exist since the application of NACT in clinical trial. The following reason might be able to explain how the discrepancy presents among different studies. First, many clinico-pathological factors, such as clinical stage, histological type and grade, physical condition, etc., can also determine the recurrence of cervical cancer. Patients subjected to the studies were at different stages and have different pathological characteristics (33). To some extent, the heterogeneity of the data will compromise the statistical significance of the study. Moreover, it is also suggested that the efficacy of NACT in different subtypes of cervical cancer can be of huge distinction, no matter short-term or long-term outcomes are investigated (6). Second, the criteria and strategy for adjuvant therapy (including the chemo-agents, dosage, cycle-length, etc.) could be different among groups, which might have great influence on the evaluation of the NACT efficacy. Third, the sample size and study design varies among each trial (6,34).
Actually, more and more attention has been drawn to the disadvantages of NACT recently. It was reported that some patients do not respond to neoadjuvant therapy at all. Hence, the delay in treatment, the development of radio-resistant cellular clones caused by NACT and so forth, could all worsen the effectiveness of the treatment (6). Another drawback of NACT is that it is thought to increase the difficulties of surgical dissection of tumor-affected pelvic tissues, in particular, which hinders the usage of robotic techniques to perform RS after NACT (35). Due to these reasons, the combination therapy of NACT and RS will not present satisfactory or better effect compared with RS alone.
Moreover, it is suggested that among the patients with pelvic lymph node metastases who were free of parametrial extension, those who received postoperative chemo-radiotherapy had significantly better DFS (P=0.021) and OS (P=0.030) than those who received no adjuvant therapy (36). However, it is also indicated that extended chemotherapy could not show advantages in terms of DFS and OS (37). Thus, we did a subgroup meta-analysis by adjuvant therapy to assess its effect on this study. There was also no significant efficacy on OS and DFS in subgroup meta-analyses conforming our results.
Ours result is consistent with many previous studies (38) by summarizing numerous previous studies and provides a quantitative assessment of the effectiveness of NACT when adding into RS treatment. We believe our study will shed new lights on future cervical cancer treatment researches. Although the sample size is small, which may possibly lead to bias in the conclusion. Further investigation would be helpful to confirm this issue by taking substantial efforts to obtain relevant data from more randomized controlled trials.
Acknowledgments
Funding: This work is supported in part by the Science and Technology Project of Chengdu (2014-HM01-00097-SF).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim K, Zang R, Choi SC, et al. Current status of gynecological cancer in China. J Gynecol Oncol 2009;20:72-6. [Crossref] [PubMed]
- Tewari KS, Sill MW, Long HJ 3rd, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 2014;370:734-43. [Crossref] [PubMed]
- Mohar A, Frías-Mendívil M. Epidemiology of cervical cancer. Cancer Invest 2000;18:584-90. [Crossref] [PubMed]
- Delgado G, Bundy B, Zaino R, et al. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol 1990;38:352-7. [Crossref] [PubMed]
- Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet 2009;105:103-4. [Crossref] [PubMed]
- Zhang J, Zhu L, Zhang Q, et al. Effects of cytokine-induced killer cell treatment in colorectal cancer patients: a retrospective study. Biomed Pharmacother 2014;68:715-20. [Crossref] [PubMed]
- Zanetta G, Lissoni A, Gabriele A, et al. Intense neoadjuvant chemotherapy with cisplatin and epirubicin for advanced or bulky cervical and vaginal adenocarcinoma. Gynecol Oncol 1997;64:431-5. [Crossref] [PubMed]
- Behtash N, Nazari Z, Ayatollahi H, et al. Neoadjuvant chemotherapy and radical surgery compared to radical surgery alone in bulky stage IB-IIA cervical cancer. Eur J Surg Oncol 2006;32:1226-30. [Crossref] [PubMed]
- Chen H, Liang C, Zhang L, et al. Clinical efficacy of modified preoperative neoadjuvant chemotherapy in the treatment of locally advanced (stage IB2 to IIB) cervical cancer: randomized study. Gynecol Oncol 2008;110:308-15. [Crossref] [PubMed]
- Cho YH, Kim DY, Kim JH, et al. Comparative study of neoadjuvant chemotherapy before radical hysterectomy and radical surgery alone in stage IB2-IIA bulky cervical cancer. J Gynecol Oncol 2009;20:22-7. [Crossref] [PubMed]
- Eddy GL, Bundy BN, Creasman WT, et al. Treatment of ("bulky") stage IB cervical cancer with or without neoadjuvant vincristine and cisplatin prior to radical hysterectomy and pelvic/para-aortic lymphadenectomy: a phase III trial of the gynecologic oncology group. Gynecol Oncol 2007;106:362-9. [Crossref] [PubMed]
- Liu H, Song J, Yang Z, et al. Effects of cytokine-induced killer cell treatment combined with FOLFOX4 on the recurrence and survival rates for gastric cancer following surgery. Exp Ther Med 2013;6:953-956. [PubMed]
- Gong L, Lou JY, Wang P, et al. Clinical evaluation of neoadjuvant chemotherapy followed by radical surgery in the management of stage IB2-IIB cervical cancer. Int J Gynaecol Obstet 2012;117:23-6. [Crossref] [PubMed]
- Katsumata N, Yoshikawa H, Kobayashi H, et al. Phase III randomised controlled trial of neoadjuvant chemotherapy plus radical surgery vs radical surgery alone for stages IB2, IIA2, and IIB cervical cancer: a Japan Clinical Oncology Group trial (JCOG 0102). Br J Cancer 2013;108:1957-63. [Crossref] [PubMed]
- Lee DW, Lee KH, Lee JW, et al. Is neoadjuvant chemotherapy followed by radical surgery more effective than radiation therapy for stage IIB cervical cancer? Int J Gynecol Cancer 2013;23:1303-10. [Crossref] [PubMed]
- Shoji T, Kumagai S, Yoshizaki A, et al. Efficacy of neoadjuvant chemotherapy followed by radical hysterectomy in locally advanced non-squamous carcinoma of the uterine cervix: a retrospective multicenter study of Tohoku Gynecologic Cancer Unit. Eur J Gynaecol Oncol 2012;33:353-7. [PubMed]
- Yin M, Zhao F, Lou G, et al. The long-term efficacy of neoadjuvant chemotherapy followed by radical hysterectomy compared with radical surgery alone or concurrent chemoradiotherapy on locally advanced-stage cervical cancer. Int J Gynecol Cancer 2011;21:92-9. [PubMed]
- Gong L, Zhang JW, Yin RT, et al. Safety and Efficacy of Neoadjuvant Chemotherapy Followed by Radical Surgery Versus Radical Surgery Alone in Locally Advanced Cervical Cancer Patients. Int J Gynecol Cancer 2016;26:722-8. [Crossref] [PubMed]
- Iwata T, Miyauchi A, Suga Y, et al. Neoadjuvant chemotherapy for locally advanced cervical cancer. Chin J Cancer Res 2016;28:235-40. [Crossref] [PubMed]
- Liu Z, Li X, Tao Y, et al. Clinical efficacy and safety of laparoscopic nerve-sparing radical hysterectomy for locally advanced cervical cancer. Int J Surg 2016;25:54-8. [Crossref] [PubMed]
- Cibula D. A Novel Perspective of Neoadjuvant Chemotherapy in Locally Advanced Cervical Cancer. Ann Surg Oncol 2016;23:2126-7. [Crossref] [PubMed]
- Panici PB, Di Donato V, Palaia I, et al. Type B versus Type C Radical Hysterectomy After Neoadjuvant Chemotherapy in Locally Advanced Cervical Carcinoma: A Propensity-Matched Analysis. Ann Surg Oncol 2016;23:2176-82. [Crossref] [PubMed]
- Lapresa M, Parma G, Portuesi R, et al. Neoadjuvant chemotherapy in cervical cancer: an update. Expert Rev Anticancer Ther 2015;15:1171-81. [Crossref] [PubMed]
- Buda A, Lissoni AA, Floriani I, et al. Long-Term Clinical Benefits of Neoadjuvant Chemotherapy in Women With Locally Advanced Cervical Cancer: Validity of Pathological Response as Surrogate Endpoint of Survival. Int J Gynecol Cancer 2015;25:1468-75. [Crossref] [PubMed]
- Ryu HS, Kang SB, Kim KT, et al. Efficacy of different types of treatment in FIGO stage IB2 cervical cancer in Korea: results of a multicenter retrospective Korean study (KGOG-1005). Int J Gynecol Cancer 2007;17:132-6. [Crossref] [PubMed]
- Mossa B, Mossa S, Corosu L, et al. Follow-up in a long-term randomized trial with neoadjuvant chemotherapy for squamous cell cervical carcinoma. Eur J Gynaecol Oncol 2010;31:497-503. [PubMed]
- Zivanovic O, Alektiar KM, Sonoda Y, et al. Treatment patterns of FIGO Stage IB2 cervical cancer: a single-institution experience of radical hysterectomy with individualized postoperative therapy and definitive radiation therapy. Gynecol Oncol 2008;111:265-70. [Crossref] [PubMed]
- Robova H, Halaska M, Pluta M, et al. The role of neoadjuvant chemotherapy and surgery in cervical cancer. Int J Gynecol Cancer 2010;20:S42-6. [Crossref] [PubMed]
- Slama J, Dundr P, Dusek L, et al. Sentinel lymph node status in patients with locally advanced cervical cancers and impact of neoadjuvant chemotherapy. Gynecol Oncol 2012;125:303-6. [Crossref] [PubMed]
- Bogani G, Cromi A, Serati M, et al. A prospective case-control study on the impact of neoadjuvant chemotherapy on surgery-related outcomes of laparoscopic radical hysterectomy. Anticancer Res 2014;34:5703-8. [PubMed]
- Kim HS, Sardi JE, Katsumata N, et al. Efficacy of neoadjuvant chemotherapy in patients with FIGO stage IB1 to IIA cervical cancer: an international collaborative meta-analysis. Eur J Surg Oncol 2013;39:115-24. [Crossref] [PubMed]
- Pareja R, Rendón GJ, Vasquez M, et al. Immediate radical trachelectomy versus neoadjuvant chemotherapy followed by conservative surgery for patients with stage IB1 cervical cancer with tumors 2cm or larger: A literature review and analysis of oncological and obstetrical outcomes. Gynecol Oncol 2015;137:574-80. [Crossref] [PubMed]
- Wang H, Zhu L, Lu W, et al. Clinicopathological risk factors for recurrence after neoadjuvant chemotherapy and radical hysterectomy in cervical cancer. World J Surg Oncol 2013;11:301. [Crossref] [PubMed]
- Lee JY, Kim YH, Kim MJ, et al. Treatment of stage IB2, IIA bulky cervical cancer: a single-institution experience of neoadjuvant chemotherapy followed by radical hysterectomy and primary radical hysterectomy. Arch Gynecol Obstet 2011;284:477-82. [Crossref] [PubMed]
- Vizza E, Corrado G, Zanagnolo V, et al. Neoadjuvant chemotherapy followed by robotic radical hysterectomy in locally advanced cervical cancer: a multi-institution study. Gynecol Oncol 2014;133:180-5. [Crossref] [PubMed]
- Behtash N, Karimi Zarchi M, Deldar M. Preoperative prognostic factors and effects of adjuvant therapy on outcomes of early stage cervical cancer in Iran. Asian Pac J Cancer Prev 2009;10:613-8. [PubMed]
- Knauer M, Haid A, Schneider Y, et al. Adjuvant extension of chemotherapy after neoadjuvant therapy may not improve outcome in early-stage breast cancer. Eur J Surg Oncol 2009;35:798-804. [Crossref] [PubMed]
- Wen H, Wu X, Li Z, Wang H, et al. A prospective randomized controlled study on multiple neoadjuvant treatments for patients with stage IB2 to IIA cervical cancer. Int J Gynecol Cancer 2012;22:296-302. [Crossref] [PubMed]


