
Comparison of clinicopathologic features and survival between patients with right-sided and left-sided stage III colon cancer
Introduction
There is an increasing incidence of right-sided colon cancer (RCC) in recent years. Previous studies have described diversities in biology and prognosis for colon cancer based on whether the primary is right-sided or left-sided (1,2). There is an ongoing debate as to whether tumor location itself represents an independent prognostic factor. Most of the previous studies have revealed that RCC would lead to an inferior prognosis (3,4). Suttie et al. reported that RCC has a worse prognosis, possibly because of more advanced staging and fewer curative resections (5). However, studies by Kwaan et al. (6) and Weiss et al. (7) demonstrated no difference in overall survival (OS) between RCC and left-sided colon cancer (LCC). In contrast, Warschkow et al. (8) came to the opposite conclusion, namely that the prognosis of localized RCC is better relative to LCC. Weiss et al. (7) reported that the relationship between long-term survival and tumor location in colon cancer is stage dependent. Retrospective analyses based on databases in some randomized controlled trials have indicated that in patients with RAS wild-type metastatic colorectal cancer (CRC), those with left-sided tumors had a markedly better prognosis than those with right-sided tumors (9). The survival diversity between RCC and LCC is still uncertain in patients without metastatic cancer. Most current studies are based on population databases from the United States or Europe (2-4,10,11). A systematic review and meta-analysis indicated that a significant difference in prognosis between RCC and LCC was identified only in Western countries, while it was inconsistent in Eastern countries (12). Hence, in the present study we aimed to perform a retrospective analysis of Chinese patients with the same stage colon cancer who underwent curative resection at our institute; the objective was to analysis if there were any differences in patient characteristics, clinicopathologic features and survival based on the location of the tumor.
Methods
Patients
A retrospective analysis of consecutive patients who underwent curative resection for histologically proven TNM stage III colon cancer at Peking Union Medical College Hospital between January 2005 and December 2012 was conducted. Patients included to this study met the following criteria: had TNM stage III colon cancer on final pathologic examination; underwent curative resection at our institution; and had complete follow-up databases. Patients with rectal cancer were excluded because the treatment is different from that of colon cancer.
The laparoscopic or open colectomy procedures were performed by the same team of surgeons according to the same oncologic principles. All patients underwent post-operative adjuvant chemotherapy with 5-fluorouracil plus oxaliplatin (FOLFOX) or capecitabine plus oxaliplatin (XELOX). All patients were included in an oncological follow-up program for at least 5 years after surgery or until death in cases of recurrence.
Patient clinical outcomes and survival status were regularly followed up. Available variables included the following: age at diagnosis; gender; tumor size; histological type; TNM classification; vascular invasion; presence carcinoma nodules; presence or absence of mucinous adenocarcinoma (MAC); lymph node ratio (LNR); surgical approach and occult blood.
RCC was defined as those tumors located in the cecum, ascending colon, hepatic flexure, and transverse colon, while LCC was defined as those tumors located in the splenic flexure, descending colon, and sigmoid. Most previous reports have the similar definition (6). The TNM classification was defined according to the criteria of the American Joint Commission on Cancer/International Union against Cancer (AJCC/UICC). The LNR was calculated by dividing the number positive lymph nodes by the number of dissected lymph nodes (13).
Statistical analysis
All data were statistically analyzed using the statistical package for the social sciences, version 17.0 (SPSS Inc., Chicago, IL, USA). The correlation was calculated using the chi-square test (for categorical variables) and Student’s t-test (for continuous variables). The Cox proportional-hazards model was used for univariate and multivariate analyses to identify the independent prognostic factors for OS and disease-free survival (DFS). OS and DFS were calculated using the Kaplan-Meier method. All tests of significance used two-sided P values at the <0.05 level.
Results
Characteristics of patients
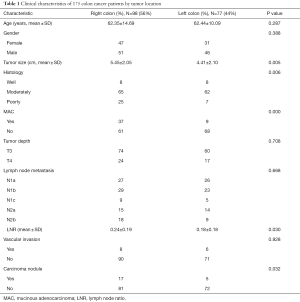
Overall, 175 patients with histologically proven stage III colon cancer who underwent curative resection between January 2005 and December 2012 were selected for study analysis. The clinicopathologic data regarding all 175 of these patients are demonstrated in Table 1. Of the 175 patients, 98 (56%) had RCC and 77 (44%) had LCC. Size was larger in right-sided than in left-sided tumors (P=0.01). RCC was found to be poorly differentiated (P=0.01), and was also more likely to develop carcinoma nodules (P=0.03). Moreover, MAC occurred more frequently in RCC (P=0.00). The mean LNR for RCC was higher than that for LCC (0.24±0.19 vs. 0.18±0.18; P=0.03). Nevertheless, no significant differences were found in terms of age at diagnosis, gender, tumor depth, lymph node stage, vascular invasion, and occult blood between RCC and LCC.
Full table
Survival analysis by tumor location
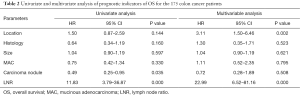
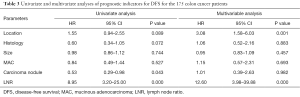
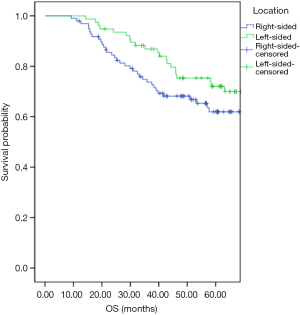
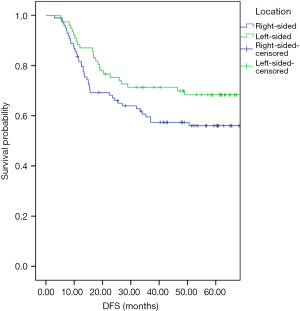
Univariate and multivariate analyses were performed to investigate independent prognostic factors for OS and DFS using the Cox proportional-hazards model (Tables 2,3). Right-sided (HR, 3.11; 95% CI, 1.50–6.46; P=0.00) and LNR (HR, 22.99; 95% CI, 6.52–81.16; P=0.00) were indicated to be independent negative prognostic factors for OS (HR, 3.08; 95% CI, 1.58–6.03; P=0.00) as well as negative prognostic factors for DFS (HR, 12.60; 95% CI, 3.98–39.88; P=0.00). The Kaplan-Meier survival analysis demonstrated that patients with RCC had poorer OS and inferior DFS than patients with LCC (Figures 1,2). The mean OS for the RCC and LCC patient groups was 89.55±9.52 and 100.32±4.93 months, respectively (P=0.02; 95% CI, 79.72–99.38 and 90.66–109.97). The 5-year OS rates of patients with RCC and those with LCC were 65.3% and 74.0%, respectively. The mean DFS for the RCC and LCC patient groups was 76.19±5.41 and 92.58±5.83 months, respectively (P=0.04; 95% CI, 65.59–86.79 and 81.18–104.01). The 5-year DFS rates for patients with RCC and those with LCC were 57.1% and 68.8%, respectively.
Full table
Full table
Discussion
Recently, several studies have focused on the analysis of colon cancer according to tumor location. There is still controversy regarding whether RCC without metastasis leads to worse survival than LCC. Through examination of the 175 cases in the present study, we found that RCC was different from LCC in both clinicopathologic features and survival. RCC was larger in size as well as poorly differentiated. Several previous studies have indicated similar findings (3,14). We analyzed a greater number of clinicopathologic features than previous studies and found that MAC occurred more frequently in RCC. As compared with LCC, RCC was more likely to develop carcinoma nodules. Furthermore, we calculated the LNR of our patients, which has been validated in a large series of colon cancer patients as an independent prognostic factor (13,15). A higher LNR value was observed in patients with RCC in this survey. No significant differences were found in terms of tumor depth, lymph node stage, vascular invasion, and occult blood. Patient age at diagnosis was similar for RCC and LCC. This finding contrasts with most recent studies, which revealed that patients who had a right-sided colectomy were older (6,10). When it comes to mortality, RCC demonstrated significantly poorer OS and inferior DFS.
In the current study, regression analysis indicated that tumor size, histology, presence of carcinoma nodules, and MAC did not impact on the OS and DFS rate, whereas tumor location and LNR were significant predictors of the OS and DFS. This suggests that a right-sided tumor location and an increasing positive LNR predict significantly worse survival. Most of the previous studies have reported a similar finding that RCC would lead to inferior prognosis (2-5,10,14). However, the results reported by Kwaan et al. and Weiss et al. demonstrated no difference in OS between RCC and LCC (6). Conversely, Warschkow et al. came to a conclusion that was completely opposed to ours, namely that the prognosis for localized RCC is better than for LCC (8).
The different clinicopathologic features and survival times between RCC and LCC suggested that carcinomas of right and left colon may be considered as different tumor entities. The reason for the relationship between long-term survival and tumor location is most likely related to tumor biology. The left-sided and right-sided colons are of different embryological origins and have different blood supplies. The right-sided colon develops from the midgut and the left-sided colon from the hindgut. Moreover, these two are reported to be physiologically different (9,16). In the present study, MAC tended to be found more often in RCC. MAC is an uncommon and rare histopathologic type of CRC. Previous studies have revealed that the oncologic behavior of MAC tumors differs from non MAC tumors, and that MAC was an independent negative prognosis factor for survival in colon cancer (17,18). A retrospective review of the pathological records of 5,817 CRC patients indicated that MAC patients had metastatic disease more frequently (19). A considerable discrepancy regarding LNR was observed between the two tumor locations. The LNR has recently emerged as an important prognostic factor in CRC, because a higher LNR was significantly associated with a shorter OS and DFS (20); this discrepancy leads to distinct outcomes. Consequently, we should administer different treatment for these two separate entities.
Previous findings have suggested that the molecular pathogenesis of the microsatellite stable phenotype in the left-sided colon was different from that in the right-sided colon (21). As a significant carcinogenesis mechanism of CRC, microsatellite instability (MSI) has a prevalence of 12–18% in sporadic CRC (22). In a randomized trial involving FOLFOX-based adjuvant chemotherapy in stage III colon cancer patients, MSI was significantly associated with RCC (23). Several studies have shown that MSI is associated with a favorable prognosis in patients with CRC (24). Nevertheless, a recent study published by Shin et al. claimed that MSI is an independent negative prognosis factor in stage II CRC (22).
We would like to acknowledge the limitations of this study. To begin with, it was a retrospective analysis of patients carried out at only a single-institution. Secondly, the sample size of our research was relatively small for a retrospective study. Thirdly, as a result of a lack of information regarding MSI, we did not analyze the relationship between tumor location, MSI, and survival. Additional randomized trials or other studies are needed to verify the relationship between tumor location and prognosis in patients with colon cancer.
Conclusions
Patients with a RCC had more negative prognostic factors, and led to inferior outcomes as compared with those with LCC. We believe that RCC should be treated differentially from LCC; patients with RCC may be considered an indication for a more aggressive therapeutic plan. In addition, the establishment of standardized management for colon cancer in terms of tumor location is needed.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.01.31). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This article was reviewed and approved by Peking Union Medical College Hospital Review Board. Written informed consent was obtained from each patient prior to initiation.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Komuro K, Tada M, Tamoto E, et al. Right- and left-sided colorectal cancers display distinct expression profiles and the anatomical stratification allows a high accuracy prediction of lymph node metastasis. J Surg Res 2005;124:216-24. [Crossref] [PubMed]
- Christodoulidis G, Spyridakis M, Symeonidis D, et al. Clinicopathological differences between right- and left-sided colonic tumors and impact upon survival. Tech Coloproctol 2010;14:S45-7. [Crossref] [PubMed]
- Meguid RA, Slidell MB, Wolfgang CL, et al. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol 2008;15:2388-94. [Crossref] [PubMed]
- Masoomi H, Buchberg B, Dang P, et al. Outcomes of right vs. left colectomy for colon cancer. J Gastrointest Surg 2011;15:2023-8. [Crossref] [PubMed]
- Suttie SA, Shaikh I, Mullen R, et al. Outcome of right- and left-sided colonic and rectal cancer following surgical resection. Colorectal Dis 2011;13:884-9. [Crossref] [PubMed]
- Kwaan MR, Al-Refaie WB, Parsons HM, et al. Are right-sided colectomy outcomes different from left-sided colectomy outcomes?: study of patients with colon cancer in the ACS NSQIP database. JAMA Surg 2013;148:504-10. [Crossref] [PubMed]
- Weiss JM, Pfau PR, O'Connor ES, et al. Mortality by stage for right- versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results--Medicare data. J Clin Oncol 2011;29:4401-9. [Crossref] [PubMed]
- Warschkow R, Sulz MC, Marti L, et al. Better survival in right-sided versus left-sided stage I - III colon cancer patients. BMC Cancer 2016;16:554. [Crossref] [PubMed]
- Tejpar S, Stintzing S, Ciardiello F, et al. Prognostic and Predictive Relevance of Primary Tumor Location in Patients With RAS Wild-Type Metastatic Colorectal Cancer: Retrospective Analyses of the CRYSTAL and FIRE-3 Trials. JAMA Oncol 2016; [Epub ahead of print]. [PubMed]
- Benedix F, Kube R, Meyer F, et al. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum 2010;53:57-64. [Crossref] [PubMed]
- Benedix F, Schmidt U, Mroczkowski P, et al. Colon carcinoma--classification into right and left sided cancer or according to colonic subsite?--Analysis of 29,568 patients. Eur J Surg Oncol 2011;37:134-9. [Crossref] [PubMed]
- Yahagi M, Okabayashi K, Hasegawa H, et al. The Worse Prognosis of Right-Sided Compared with Left-Sided Colon Cancers: a Systematic Review and Meta-analysis. J Gastrointest Surg 2016;20:648-55. [Crossref] [PubMed]
- Garcia B, Guzman C, Johnson C, et al. Trends in lymph node excision and impact of positive lymph node ratio in patients with colectomy for primary colon adenocarcinoma: Population based study 1988 to 2011. Surg Oncol 2016;25:158-63. [Crossref] [PubMed]
- Huang CW, Tsai HL, Huang MY, et al. Different clinicopathologic features and favorable outcomes of patients with stage III left-sided colon cancer. World J Surg Oncol 2015;13:257. [Crossref] [PubMed]
- Amri R, Klos CL, Bordeianou L, et al. The prognostic value of lymph node ratio in colon cancer is independent of resection length. Am J Surg 2016;212:251-7. [Crossref] [PubMed]
- van der Post S, Hansson GC. Membrane protein profiling of human colon reveals distinct regional differences. Mol Cell Proteomics 2014;13:2277-87. [Crossref] [PubMed]
- Park JS, Huh JW, Park YA, et al. Prognostic comparison between mucinous and nonmucinous adenocarcinoma in colorectal cancer. Medicine (Baltimore) 2015;94:e658 [Crossref] [PubMed]
- Nitsche U, Friess H, Agha A, et al. Prognosis of mucinous and signet-ring cell colorectal cancer in a population-based cohort. J Cancer Res Clin Oncol 2016;142:2357-66. [Crossref] [PubMed]
- Hugen N, van de Velde CJ, de Wilt JH, et al. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol 2014;25:651-7. [Crossref] [PubMed]
- Zhang MR, Xie TH, Chi JL, et al. Prognostic role of the lymph node ratio in node positive colorectal cancer: a meta-analysis. Oncotarget 2016;7:72898-907. [PubMed]
- Takahashi Y, Sugai T, Habano W, et al. Molecular differences in the microsatellite stable phenotype between left-sided and right-sided colorectal cancer. Int J Cancer 2016;139:2493-501. [Crossref] [PubMed]
- Shin US, Cho SS, Moon SM, et al. Is microsatellite instability really a good prognostic factor of colorectal cancer? Ann Coloproctol 2014;30:28-34. [Crossref] [PubMed]
- Sinicrope FA, Mahoney MR, Smyrk TC, et al. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J Clin Oncol 2013;31:3664-72. [Crossref] [PubMed]
- Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23:609-18. [Crossref] [PubMed]


