
Metabolic sculpting of the mitochondria, cell signaling and the cancer phenotype
Otto Warburg’s work on cellular respiration that defines the cancer phenotype awarded him the Nobel Prize in Physiology/Medicine in 1931 (1,2). Warburg observed that cancer cells exhibit anaerobic respiration, where glucose is oxidized to lactate rather than mitochondrial aerobic respiration, where glucose is oxidized to CO2. This cascade of events is triggered in cancer cells even when there was sufficient oxygenation to allow for aerobic oxidation. High levels of active hypoxia inducible factor 1 alpha subunit (HIF-1α) and the activation of hypoxic response elements is the driving force behind the phenotypical change, however if this ‘hypoxic’ phenotype is the result of low oxygen levels or due to ‘chemical hypoxia’ is unclear (3-8).
Lipopolysaccharides (LPS) treatment of macrophages not only stimulates an immune response, but leads to alterations in mitochondrial metabolism, including the inhibition of complexes II and III (9). Macrophages treated with LPS have altered steady levels of Krebs cycle metabolites, as metabolic flux is constrained at complexes II and III (10,11). LPS treatment of macrophages then leads to an increase in the steady state mitochondrial reactive oxygen species (ROS) production (12). The underlying mechanism of macrophage metabolic changes that are induced by LPS signaling, and is relationship to the Warburg effect, has been explored, but is still not clearly understood (10,11,13).
The recent paper in Cell by Mills and co-workers examined the relationship of mitochondrial respiration, generation of ROS, the steady state levels of HIF-1α and the generation of both pro/anti-inflammatory cytokines in macrophages activated by LPS (14). Mills et al. demonstrated that elevated mitochondrial succinate increases mitochondrial succinate oxidation and that this in-turn hyperpolarizes mitochondria, elevating ROS levels, increases HIF-1α activation and so triggers wide spread changes in gene expression via hypoxia response elements (HRE), including the alteration in the expression levels of a range of pro/anti-inflammatory genes. In addition, the authors suspect that macrophage gene expression may be subject to epigenetic reprogramming, a process that is a common feature of many human cancers, especially in gliomal isocitrate dehydrogenase 1 (IDH1) mutants. In cancer cells harboring mutations in succinate dehydrogenase (SDH) genes or in gliomal IDH1 mutants, inhibition of 2-oxoglutarate-dependent histone and DNA demethylase enzymes, results in epigenetic-based alterations of gene expression, such epigenetic changes have been observed in macrophages (15,16).
Mills et al. demonstrate that mitochondrial ROS generation is a key determinant of the macrophage inflammatory phenotype. They further conclude that it is the oxidation of succinate that is central to the response to LPS. Mills et al. clearly couple the steady state levels of metabolic substrates to the alteration of cytokine generation, following an immunological signal for the first time. This macrophage study which shows how alterations in cellular metabolism profoundly alters cellular responses to cytokines and to cellular generation of cytokines may be applicable to other cell types, and may be transferable to pathologies, like cancer.
In macrophages activation by LPS alterations in succinate oxidation leads to hyperpolarization of mitochondria, and such increases in the steady state mitochondrial membrane potential causes an increase in the generation of both superoxide and hydrogen peroxide (17-19). Elevated peroxide levels increase the steady state levels of active HIF-1α, as peroxide is a highly effective inhibitor of the two oxygen dependent enzymes that deactivate HIF-1α; prolyl hydroxylases (PHDs) (5,7,20,21) and factor inhibiting HIF (FIH) (4,22). HIF-1α is a transcription factor that, in conjunction with histone acetyltransferase P300 (EP300) and aryl hydrocarbon receptor nuclear translocator (ARNT), controls the expression of genes involved in the response to hypoxia. Under normoxia, HIF-1α is hydroxylated at either an asparaginyl residue by FIH or of proline residues by PHDs, resulting in its inactivation. Both FIH and PHDs require O2, Fe(II), ascorbate, and 2-oxoglutarate as cosubstrates to exert their function. The Fe/2-oxoglutarate binding site is open and can react with peroxides, that generate a highly oxidizing oxy-ferryl species that deactivates these enzymes (23). Thus peroxides can increase the levels of active HIF-1α.
In addition to low oxygen levels leading to active HIF-1α and triggering the hypoxic phenotype, it has long been known that a number of reagents can trigger ‘chemical hypoxia’, by supporting high intracellular levels of active HIF-1α. Chemical hypoxia can be defined as the initiation and maintenance the physiological cellular response to low oxygen levels, independent of the actual levels of oxygen, coinage by Freeman and Gibson (24). PHDs and FIH are inhibited by removal of the iron atom by chelators [or substitution with cobalt, by high levels of cytoplasmic succinate (product inhibition) or low levels of cytosolic 2-oxoglutarate (substrate deprivation), and of course by oxidation with ROS (3,4,7,8,25,26)]. Mills et al. have added mitochondrial succinate to the range of compounds that are able to induce ‘chemical hypoxia’.
Based on a number of elegant experiments, including usage of the mitochondrially targeted antioxidant MitoQ (27), the authors demonstrate that mitochondrial ROS generation by succinate is due to reversed electron transport, where the high mitochondrial membrane potential and high ubiquinol levels causes back flow of electrons into complex I, giving rise to radical species, which then reduce molecular oxygen to super oxide, and subsequently to hydrogen peroxide (28).
Mitochondria, hypoxia and gliomas
Glioblastoma multiforme (GBM), the most common and aggressive brain cancer characterized by high degree of hypoxia, and are further distinguished by differing gene expression giving rise to major sub-type classifications. Notably, grade III glioma differ by their expression of IDH1 linked to a mutation in a single amino acid (R132, typically R132H). IDH1 mutations have also been found in a number of cancer types in addition to glioma, including acute myelogenous leukemia, cholangiocarcinoma, cartilaginous tumors, prostate cancer, papillary breast carcinoma, acute lymphoblastic leukemia, angioimmunoblastic T-cell lymphoma, and primary myelofibrosis.
In light of Mills et al., we examined changes in the steady state levels of mRNA and patient survival, in GBM, and in IDH1 mutant and IDHA wild-type grade III glioma to see if we could observe any evidence for mitochondrial succinate/SDH impacting the cancer phenotype and patient outcome. Approximately 75% of grade III glioma harbor IDH1 mutations and the major difference between the R132 mutant and wild-type is patient survival. The cbioportal (29,30) grade III gliomal dataset, where there is both survival data and mRNA levels (RNA Seq V2 RSEM), indicates that the median survival patients harboring IDH1 mutations is 43.9 months and yet in patients with wild-type IDH the median survival time is only 19.9 months. This clearly presents a paradox. There is an evolutionary selection pressure for glioma with a mutated IDH1, but the more aggressive wild-type IDH grade III gliomas are faster growing. In a heterogeneous population one would expect that the rapidly growing cells to become dominant, yet in >75% of patients this is not the case. The findings of Mills et al., provides insight as to why the less aggressive IDH1 mutant glioma are so common. Alterations in cellular metabolism give rise to HIF-1α-dependent alterations in cytokine signaling, and this alteration of cytokine signaling in IDH1-mutant glioma could allow this phenotype to evade immune-surveillance at the initial stages of tumor growth, giving these cells a positive evolutionary selection pressure advantage.
Wild-type IDH1 form heterodimers with IDH1 mutants conferring a gain-of-function and allowing the Mut/WT heterodimer to convert 2-oxoglutarate to R(−)-2-hydroxyglutarate (R-2-HD). In gliomas, R-2-HD levels are elevated in those with a IDH1 mutation and have been shown to drive increased methylation. It has also been shown that CD8+ T-lymphocytes accumulate 2-HD in response to T-cell receptor triggering (31). This increases to millimolar levels in physiological oxygen conditions, via a HIF-1α-dependent mechanism. Unlike IDH1 mutants in these cells it is the S-2-HD isomer that predominates over R-2-HD (4:1) and the S-2-HD is generated from 2-oxoglutarate via lactate dehydrogenase A (LDHA), which is upregulated by HIF-1α. It appears that R-2-HD is generated, also via a HIF-1α-dependent mechanism, in cells lacking either IDH1 or IDH2 mutations from 2-oxoglutarate through a truncated, noncarboxylating reductive reaction, by IDH2. Increases in both 2-HD stereoisomers can inhibit a range of 2-oxoglutarate-dependent enzymes, histone and DNA demethylase enzymes, resulting in epigenetic alterations in gene expression levels.
We examined the cBioPortal grade III gliomal dataset of survival statistics and mRNA levels in 42 R132X IDH1 mutant grade III glioma, 82 wild-type grade III glioma and of 110 GBM (provisional dataset) (28,29). We examined a range of gene mRNA transcript levels comparing patient survival time between the 50% low and 50% high mRNA levels of genes that can influence succinate and 2-oxoglutarate levels. As an internal control marker of active HIF-1α, we used the lysyl oxidase (LOX) gene which is under control of a hypoxia response element (14), and other genes under control by HRE.
Table 1 shows the statistically significant patient survival rates, using Cox’s regression model of the Kaplan-Meier survival curves (32), for 50% high and 50% low levels of LOX, IDH1, SDHA, SDHB, glutamate dehydrogenase 1 (GLUD1) and GLUD2. SDHA&B and GLUD1&2 datasets generated by the addition of the two individual 2(log2) mRNA levels.
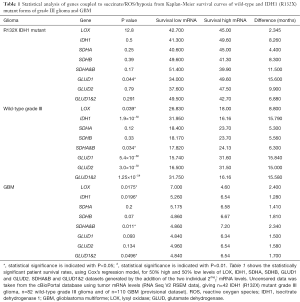
Full table
In contrast to the GBM and wild-type grade III glioma, it is clear that the survival time of patients with the (R132X) IDH1 mutants is not linked to either physiological or chemical hypoxia. We found no differences in patient outcomes when comparing tumors with low or high expression levels of LOX or other HRE controlled genes. In GBM and wild-type grade III glioma, high LOX levels are a risk factor and poor patient outcomes was statistically correlated with other markers of active HIF-1α, including vascular endothelial growth factor A (VEGFA) and PKD2. The mRNA levels of EP300 and HIF-1α, part of the active hypoxic response complex, are negatively correlated with LOX, and high levels proteins are linked to improved patient outcome, in both wild-type grade III glioma and in GBM. This suggests that expression levels of EP300 and HIF-1α, are under negative feedback control by active HIF-1α in the WT grade III and in the GBM tumors. In the R132 IDH1 mutants we found no difference in patient survival in the levels of IDH1, SDHA, SDHB, SDHA&B, GLUD2 or GLUD1&2. There as a statistically significant decrease in patient survival if their tumors had low levels of GLUD1, but no other enzyme system that modulated succinate/2-oxyglutarate had any significant effect on patient survival outcome.
In the wild-type grade III glioma we find evidence that metabolic processes are correlated with active HIF-1α, and that this is unambiguously linked to patient survival. In low grade glioma, low levels of either SDHA or SDHB independently have no effect on survival. However we do observe differences in patient survival in tumors with either high or low levels of SDHA and SDHB, the core of the active complex II. Low SDH activity in tumors, and thus high levels of succinate, indicates a poor prognosis. In the grade III glioma, high mitochondrial respiratory flux caused by high levels of IDH1 and low levels of both GLUDs is linked to poor outcomes. This is because high IDH1, low SDHA/SDHB and therefore low GLUD1/GLUD2 will manifest high levels of NADH-linked mitochondrial flux, with the concomitant mitochondrial membrane potential driven generation of super oxide/hydrogen peroxide. This ROS will deactivate PHD’s and FIH, increasing steady state active HIF-1α and driving the ‘Warburg’ transformation. Coupled with this peroxide drive ‘chemical hypoxia’ the phenotype will also have high levels of succinate, caused by low levels of SDHA/SDHB, which in turn will inhibit PHD’s and FIH. Low levels of either or both GLUDs correlates with poor outcome and in these glioma suggest that flux from glutamate to 2-oxoglutamate is a significant pathway for the generation of 2-oxoglutamate.
Patient outcome in GBM follows a similar pattern to that observed in grade III wild-type glioma, but in these tumors it appears they are at the extreme end of the ‘Warburg’ phenotype. LOX, IDH1 and SDHA/SDHB and GLUD1/SDH2 levels are statistically significant risk factors in GBM. High mitochondrial flux and associated ROS, elevated succinate and low GLUD levels all drive an increase in active HIF-1α and induce ‘chemical hypoxia’, driving the ‘Warburg effect’, and triggering the activation of HIF dependent genes. In this GBM dataset we observe statistically significant correlations (P<0.05) between the classical hypoxia genes solute carrier family 2 member 1 (SLC2A1) (GLUT1) solute carrier family 2 member 3 (SLC2A3) (GLUT3), hexokinase 2 (HK2), LDHA and VEGFA, and higher levels of the mRNA transcripts.
Other genes linked to ‘chemical hypoxia’ in glioma
Examination of gliomal mRNA levels, in light of Mills et al., prompted us to examine the role of genes that could be responsible for inducing ‘chemical hypoxia’ either by the generation of peroxides or via the chelation of iron, such as O-phenanthroline or desferrioxamine.
We had previously noted that the hydrogen peroxide generating enzyme monoamine oxidase B (MAOB) is highly expressed in GBM, and that levels of MAOB are highly correlated with tumor grade and tumor HIF-1α levels (33). We observed statistically significant correlation (P<0.02) between the 25% of GBM with low levels of MAOB and the majority 75% with highly elevated levels of MAOB transcripts.
Lactotransferrin (LTF) is normally found in secretory fluids, milk, saliva, tears, and in nasal secretions, however we did find significant mRNA levels in wild-type grade III glioma. The median survival of the 50% of patients with tumors expressing the lowest levels of LTF was 32.3 months compared with the 50% of tumors with the highest levels of LTF having a median survival of only 15 months (P=4×10−6). LTF gave the greatest difference in patient outcome, for a 50% high: low mRNA levels, of all the genes we examined in these wild-type grade III gliomas.
Autism and mitochondrial ‘chemical hypoxia’
Disorders of energy metabolism have been associated with autism spectrum disorders (ASD) and indeed, many ASD subjects appear to exhibit aberrant mitochondrial function as well as additional immunological disturbances. Individuals with ASD (and some family members) show increased autoimmunity, altered cellular immunity and unusual patterns of cytokine expression (34). The ASD phenotype appears to have all the features of mitochondrial ROS ‘chemical hypoxia’ including elevated lactate, low levels of antioxidant defenses and high ROS. Some ASD subjects have elevated plasma succinate and defects in complex I or in complex V, supporting a succinate/reversed electron transport mechanism for mitochondrial generated ROS. In fact, urine analysis of children with ASD present higher levels of succinate compared with healthy age matched controls (35). Finally, the ROS ‘chemical hypoxia’ effect on cytokine production observed in macrophages is pro-inflammatory, a common feature of ASD subjects. A comparison of cerebellar tissue of individuals with ASD of show a decrease in glutathione peroxidase, glutathione-S-transferase and glutamate cysteine ligase activity, all involved in oxidant defense, compared to age-matched controls (36).
Muscle biopsies of children with ASD suspected to have mitochondrial defects indicated that these are common, with mitochondrial complex I defects found in 50% of the affected cohort. Combined complex I and III defects were present in 18% and complex V defects in 14% of subjects (37). Mitochondrial ROS generation is higher in ASD than in controls and oxidative damage to mtDNA has been found to be elevated in ASD (38). Plasma lactate is linked to the ASD phenotype with both high lactic acid levels and an elevated lactate to pyruvate ratio being reported (39). Brain magnetic resonance spectroscopic imaging of ASD affected adults and children, along with age matched controls, has indicated that lactate generation is higher in ASD subjects, and that high lactate generation is focal, and not global, with elevated lactate being detected most frequently within the cingulate gyrus (40), an area revealed by fMRI studies to be hyperactivated in ASD subjects (41).
Conclusions
The connection between mitochondrial ROS generation, activation of the hypoxic response element by active HIF-1α and an alteration in cytokine expression in macrophages is demonstrated by Mills et al., in macrophages is unquestionable. We are a step closer to understanding that metabolic sculpting can give rise to ROS and drive changes in gene expression, demonstrating that gene expression can bring forth a particular phenotype that sculpts the steady state levels of metabolites within cells. The opposite is also true in that the steady state levels of metabolites can drive gene expression and generate a phenotype. Even minor alterations in the rate at which succinate is oxidized by macrophage mitochondria alters not only these cells metabolism, but how they exchange information with other cells, via changes in the cytokines they generate and in how they respond to signaling molecules. A role for mitochondria in immune signaling and in the cellular immune response is yet another string to the bow for this organelle, which is central to so many aspects of physiology and pathophysiology.
The impact of Mills et al., and others like it is clear—whether cellular respiration related to glycolysis and the triggering of such events is only a symptom of cancer or the source and further defining cancer as a metabolic disease (which Warburg believed was the case) that brings about an abnormal signal autonomy layered with the complexity of the genetic expression will continue to be the focus of many future scientific exploration.
Acknowledgments
We thank the American Brain Tumor Association, Donna and Kenneth Peak, The Kenneth R. Peak Foundation, The John S. Dunn Foundation, The Taub Foundation, The Pauline Sterne Wolff Memorial Foundation, Kelly Kicking Cancer Foundation, The Methodist Hospital Foundation, and The Veralan Foundation.
Funding: None
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Hao Feng (Experimental Surgical Research, Department of General, Visceral, Transplant, Vascular and Thoracic Surgery, Hospital of the LMU Munich, Munich, Germany).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.01.33). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Koppenol WH, Bounds PL, Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer 2011;11:325-37. [Crossref] [PubMed]
- Warburg O. On the origin of cancer cells. Science 1956;123:309-14. [Crossref] [PubMed]
- López-Lázaro M. Dual role of hydrogen peroxide in cancer: possible relevance to cancer chemoprevention and therapy. Cancer Lett 2007;252:1-8. [Crossref] [PubMed]
- Masson N, Singleton RS, Sekirnik R, et al. The FIH hydroxylase is a cellular peroxide sensor that modulates HIF transcriptional activity. EMBO Rep 2012;13:251-7. [Crossref] [PubMed]
- Niecknig H, Tug S, Reyes BD, et al. Role of reactive oxygen species in the regulation of HIF-1 by prolyl hydroxylase 2 under mild hypoxia. Free Radic Res 2012;46:705-17. [Crossref] [PubMed]
- Oliver L, Olivier C, Marhuenda FB, et al. Hypoxia and the malignant glioma microenvironment: regulation and implications for therapy. Curr Mol Pharmacol 2009;2:263-84. [Crossref] [PubMed]
- Pan Y, Mansfield KD, Bertozzi CC, et al. Multiple factors affecting cellular redox status and energy metabolism modulate hypoxia-inducible factor prolyl hydroxylase activity in vivo and in vitro. Mol Cell Biol 2007;27:912-25. [Crossref] [PubMed]
- Xia M, Huang R, Sun Y, et al. Identification of chemical compounds that induce HIF-1alpha activity. Toxicol Sci 2009;112:153-63. [Crossref] [PubMed]
- El Kasmi KC, Stenmark KR. Contribution of metabolic reprogramming to macrophage plasticity and function. Semin Immunol 2015;27:267-75. [Crossref] [PubMed]
- Kelly B, O'Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res 2015;25:771-84. [Crossref] [PubMed]
- Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013;496:238-42. [Crossref] [PubMed]
- Bulua AC, Simon A, Maddipati R, et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J Exp Med 2011;208:519-33. [Crossref] [PubMed]
- Palsson-McDermott EM, Curtis AM, Goel G, et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab 2015;21:65-80. [Crossref] [PubMed]
- Mills EL, Kelly B, Logan A, et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016;167:457-470.e13. [Crossref] [PubMed]
- Yang M, Pollard PJ. Succinate: a new epigenetic hacker. Cancer Cell 2013;23:709-11. [Crossref] [PubMed]
- Letouzé E, Martinelli C, Loriot C, et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell 2013;23:739-52. [Crossref] [PubMed]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 2009;417:1-13. [Crossref] [PubMed]
- Suski JM, Lebiedzinska M, Bonora M, et al. Relation between mitochondrial membrane potential and ROS formation. Methods Mol Biol 2012;810:183-205. [Crossref] [PubMed]
- Skulachev VP. Why are mitochondria involved in apoptosis? Permeability transition pores and apoptosis as selective mechanisms to eliminate superoxide-producing mitochondria and cell. FEBS Lett 1996;397:7-10. [Crossref] [PubMed]
- Rabie T. Hypoxic Response in Senescent Brain Is Impaired: Possible Contribution to Neurodegeneration. In: Hayat MA. editor. Tumor Dormancy, Quiescence, and Senescence, Volume 1: Aging, Cancer, and Noncancer Pathologies. Dordrecht: Springer Netherlands, 2013:181-94.
- Marinho HS, Real C, Cyrne L, et al. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol 2014;2:535-62. [Crossref] [PubMed]
- Wang E, Zhang C, Polavaram N, et al. The role of factor inhibiting HIF (FIH-1) in inhibiting HIF-1 transcriptional activity in glioblastoma multiforme. PLoS One 2014;9:e86102 [Crossref] [PubMed]
- Mantri M, Zhang Z, McDonough MA, et al. Autocatalysed oxidative modifications to 2-oxoglutarate dependent oxygenases. FEBS J 2012;279:1563-75. [Crossref] [PubMed]
- Freeman GB, Nielsen P, Gibson GE. Monoamine neurotransmitter metabolism and locomotor activity during chemical hypoxia. J Neurochem 1986;46:733-8. [Crossref] [PubMed]
- Bonello S, Zähringer C. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler Thromb Vasc Biol 2007;27:755-61. [Crossref] [PubMed]
- Li YN, Xi MM, Guo Y, et al. NADPH oxidase-mitochondria axis-derived ROS mediate arsenite-induced HIF-1α stabilization by inhibiting prolyl hydroxylases activity. Toxicol Lett 2014;224:165-74. [Crossref] [PubMed]
- Lee S, Tak E, Lee J, et al. Mitochondrial H2O2 generated from electron transport chain complex I stimulates muscle differentiation. Cell Res 2011;21:817-34. [Crossref] [PubMed]
- Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem 2002;80:780-7. [Crossref] [PubMed]
- Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013;6:pl1. [Crossref] [PubMed]
- Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2:401-4. [Crossref] [PubMed]
- Tyrakis PA, Palazon A, Macias D, et al. S-2-hydroxyglutarate regulates CD8+ T-lymphocyte fate. Nature 2016;540:236-41. [Crossref] [PubMed]
- Christensen E. Multivariate survival analysis using Cox's regression model. Hepatology 1987;7:1346-58. [Crossref] [PubMed]
- Sharpe MA, Baskin DS. Monoamine oxidase B levels are highly expressed in human gliomas and are correlated with the expression of HiF-1α and with transcription factors Sp1 and Sp3. Oncotarget 2016;7:3379-93. [PubMed]
- Goines PE, Ashwood P. Cytokine dysregulation in autism spectrum disorders (ASD): possible role of the environment. Neurotoxicol Teratol 2013;36:67-81. [Crossref] [PubMed]
- Emond P, Mavel S, Aïdoud N, et al. GC-MS-based urine metabolic profiling of autism spectrum disorders. Anal Bioanal Chem 2013;405:5291-300. [Crossref] [PubMed]
- Gu F, Chauhan V, Chauhan A. Impaired synthesis and antioxidant defense of glutathione in the cerebellum of autistic subjects: alterations in the activities and protein expression of glutathione-related enzymes. Free Radic Biol Med 2013;65:488-96. [Crossref] [PubMed]
- Shoffner J, Hyams L, Langley GN, et al. Fever plus mitochondrial disease could be risk factors for autistic regression. J Child Neurol 2010;25:429-34. [Crossref] [PubMed]
- Napoli E, Wong S, Giulivi C. Evidence of reactive oxygen species-mediated damage to mitochondrial DNA in children with typical autism. Mol Autism 2013;4:2. [Crossref] [PubMed]
- Correia C, Coutinho AM, Diogo L, et al. Brief report: High frequency of biochemical markers for mitochondrial dysfunction in autism: no association with the mitochondrial aspartate/glutamate carrier SLC25A12 gene. J Autism Dev Disord 2006;36:1137-40. [Crossref] [PubMed]
- Goh S, Dong Z, Zhang Y, et al. Mitochondrial dysfunction as a neurobiological subtype of autism spectrum disorder: evidence from brain imaging. JAMA Psychiatry 2014;71:665-71. [Crossref] [PubMed]
- Dichter GS, Felder JN, Bodfish JW. Autism is characterized by dorsal anterior cingulate hyperactivation during social target detection. Soc Cogn Affect Neurosci 2009;4:215-26. [Crossref] [PubMed]


