
The role of androgen receptor signaling pathways in bladder cancer
IntroductionOther Section
- Introduction
- Expression patterns of the AR in bladder cancer
- The role of the AR signaling pathway in tumorigenesis and cancer progression in bladder cancer
- AR signaling: a novel therapeutic target in bladder cancer
- Conclusions
- Acknowledgments
- Footnote
- References
Bladder cancer is estimated to be the 9th most common malignancy worldwide (1). Approximately 90% of bladder cancers have the histology of urothelial carcinoma (2). More than 70% of urothelial carcinomas of the urinary bladder (UBCs) are diagnosed as non-muscle invasive bladder cancers (NMIBCs) at initial diagnosis, while 20% to 30% of UBCs are muscle invasive bladder cancers (MIBCs) which require more comprehensive treatments (3). MIBC finally progresses into metastatic diseases despite radical surgery, and it should be treated with systemic chemotherapy (4). For patients with metastatic UBC, cisplatin-based chemotherapy, such as gemcitabine-cisplatin or MVAC regimen, has been regarded as a gold standard therapeutic option over the last few decades (4). However, response rates are poor with high resistance to these agents, and there are no approved targeted drugs for treating advanced UBC (5). Therefore, many researchers have explored novel therapeutic strategies by discovering potential target molecules, including angiogenesis-related factors, receptor tyrosine kinase, and immune checkpoint molecules, to improve the prognosis of patients with cisplatin-resistant UBC.
Interestingly, several epidemiologic data have shown that the risk of bladder cancer and its aggressiveness are significantly different according to the sex (6). Male patients show three- to four-fold higher incidence of UBC than female patients, while female patients show more aggressive features of UBC than male patients (6). In rat models of bladder cancer, male rats showed a significantly higher incidence of spontaneous occurring tumors or N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN)-induced tumors in the urinary bladder than female rats (7,8). Different environmental and life-style factors, such as the risk of exposure to industrial chemicals and the tendency of cigarette smoking, have been primarily suggested as the causes of these sex disparities. However, different characteristics of bladder cancer according to the sex are persistently observed after adjusting for the other carcinogenic factors (9).
Different sex hormone environments according to the sex have been recently proposed as an emerging underlying mechanism of sex discrepancy in UBC patients (6). Particularly, the androgen receptor (AR) signaling pathway has been suggested as a crucial factor that is involved in the etiology and tumor progression of UBC as well as in resistance to conventional systemic chemotherapy (9,10). Accordingly, UBC is being recognized as a type of endocrine-related cancer (11). However, precise molecular mechanisms of how AR signaling regulates growth and progression of UBC and what is the clinical meaning of previous evidence on the association between AR signaling and tumor biology of UBC are still unclear.
Here, we thoroughly review the preclinical and clinical evidences supporting the hypothesis that the AR signaling pathway crucially regulates growth and progression of UBC. We also highlight the potential usefulness of AR targeting treatment as a novel therapeutic strategy for patients with advanced UBC.
Expression patterns of the AR in bladder cancerOther Section
- Introduction
- Expression patterns of the AR in bladder cancer
- The role of the AR signaling pathway in tumorigenesis and cancer progression in bladder cancer
- AR signaling: a novel therapeutic target in bladder cancer
- Conclusions
- Acknowledgments
- Footnote
- References
AR is a steroid hormone nuclear receptor regulated by androgens, and it acts as a DNA-binding transcription factor in the cells (12). Mechanistically, testosterone/5α-dihydrotestosterone (DHT) binds to the ligand domain of AR, inducing AR conformational change, and it translocates into the nucleus and subsequently activates transcription of several target genes (ligand-dependent pathway) (13). In addition, growth factors or cytokines other than androgens also trigger AR activities via a common oncogenic signaling pathway, such as PI3/AKT and ERK/MAPK (non-ligand dependent pathway) (13). Although AR and its target molecules primarily play a crucial role in growth and proliferation of prostate cancer cells (14), they have been found to be associated with tumor initiation and progression of other hormone-related cancers, including lung, kidney and breast cancers (15-17). Mikkonen et al. reported that androgen administration significantly upregulated AR expression in murine lung and altered gene expression patterns in a human lung cancer cell line, A459 (15). Moreover, various types of human lung cancers showed AR positivity by immunohistochemistry (IHC) in tissue microarray (TMA) (15). In renal cell carcinoma, one of the most common types of urological malignancies, AR-induced HIF2α/VEGF signaling accelerated the progression of RCC and genetic alteration of AR expression significantly influenced cell proliferation, migration, and invasion in various RCC models both in vitro and in vivo (18). The study by He et al. (18) suggested the AR signaling pathway as a novel key player that aggravates growth and tumor progression and that it is a novel therapeutic target in RCC.
In bladder cancer, IHC studies revealed that AR expression was positive in surgical specimens, and it ranged from 13% to 55%, without significant differences in AR expression between male and female samples, as shown in Table 1 (19-25). However, normal urothelial tissues showed higher levels of AR expression compared to matched neoplastic tissues. In the study by Miyamoto et al. (20), the expression of AR was assessed by IHC in 188 UBC specimens and 141 benign bladder samples, and benign tissues showed significantly higher AR positive rates than UBC tissues (80.1% versus 42.0%, respectively). Conversely, a significant decrease in AR protein expression was observed in high-grade or muscle-invasive tumors of the urinary bladder. Boorjian and colleagues also reported that 75% of superficial tumors were AR positive compared with 21.4% of muscle-invasive tumors, indicating that AR down-regulation was associated with tumor progression (21).
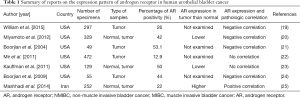
Full table
However, other studies provided conflicting results, and they showed no AR expression in normal urothelial tissues or up-regulated AR expression in high-grade and muscle-invasive tumors. Large multi-institutional data from the Toronto & Dallas cohort revealed that AR expression was actually uncommon in specimens of 472 patients with UBC (N=61/472; 12.9%), and there was no difference in the pathological grade, stage and gender as well as in the estimates of oncological outcomes according to AR positivity (22). Discrepancies between previous studies and the study by Mir et al. (22) may be attributable to technical and procedural variations, including different experimental conditions of TMA and IHC, different types of primary antibodies and different scoring criteria for AR expression levels in UBC specimens. Kauffman and colleagues investigated the expression patterns of AR and AR co-regulatory proteins (JMJD2A and LSD1) in specimens of 72 UBC patients who underwent radical cystectomy (23). Interestingly, the expression levels of JMJD2A and AR proteins on IHC were remarkably lower in UBC tissue specimens compared to benign tissue specimens. Moreover, all three markers (JMJD2A, LSD1 and AR) were significantly down-regulated by tumor aggressiveness, such as the presence of muscle invasion, extravesical extension and lymph node invasion. Similar to bladder cancer specimens, various human bladder cancer cell lines also expressed AR as well as AR co-activators. For instance, two bladder cancer cell lines (TCC-SUP and UMUC3) significantly expressed AR and its co-activators NCOA1, NCOA2, NCOA3, CREBBP, and EP300 (24).
Taken together, these evidences suggest that human bladder tissues, non-neoplastic and neoplastic tissues, have distinct expression patterns of the AR signaling pathway, and therefore, the AR signaling pathway can be significantly associated with the etiology, biology and prognosis of UBC.
The role of the AR signaling pathway in tumorigenesis and cancer progression in bladder cancerOther Section
- Introduction
- Expression patterns of the AR in bladder cancer
- The role of the AR signaling pathway in tumorigenesis and cancer progression in bladder cancer
- AR signaling: a novel therapeutic target in bladder cancer
- Conclusions
- Acknowledgments
- Footnote
- References
Many preclinical studies have revealed that AR signaling was associated with bladder carcinogenesis and cancer progression. Surgical castration or chemical castration by anti-androgen or LHRH agonist significantly reduced the occurrence of BBN-induced bladder cancer in male rats (26). More interestingly, BBN-induced bladder carcinoma developed in 90% of wild-type male mice and in 40% of wild-type female mice, whereas bladder carcinogenesis was not induced by BBN treatment in AR knockout (ARKO) male and female mice. When ARKO mice were supplemented with DHT, 25% of ARKO mice eventually developed BBN-induced bladder tumors (27). Furthermore, Hsu and colleagues found that mice lacking only urothelial AR had a lower rate of BBN-induced bladder tumorigenesis and better survival outcomes compared to wild type mice (28). They provided the first in vivo evidence that AR in urothelium may play a crucial role in carcinogenesis and prognosis of UBC. Additionally, AR signaling is associated with drug resistance in bladder cancer. The research team at the Kyushu University showed that up-regulated AR expression in several bladder cancer cell lines was significantly associated with doxorubicin resistance, and therefore, bladder cancer cells were vulnerable to doxorubicin by AR blockade or AR knockdown (29).
More importantly, clinical evidence has recently suggested that the AR signaling pathway can be associated with oncological outcomes of UBC. Nam et al. analyzed 169 patients with NMIBC and they showed that AR expression was significantly associated with lower recurrence rates [hazard ratio (HR) =0.50] after adjusting for other clinical factors (30). Izumi and colleagues reviewed 20,328 patients with prostate cancer, and they finally identified 239 (1.2%) patients who were concurrently diagnosed with primary UBC (31). Interestingly, the authors found that patients who were treated with androgen deprivation therapy (ADT) for prostate cancer had remarkably lower rates of bladder cancer recurrence compared to those who did not receive ADT. Moreover, ADT was identified as a significant predictor of recurrence of UBC in the multivariate analysis (HR =0.29). This study provides the first clinical evidence on the preventive effects of ADT on bladder cancer recurrence. The same research group also performed IHC on 72 NMIBC specimens and 42 matched normal tissues, and confirmed that patients with AR-positive cancer showed lower rates of disease recurrence than those with AR-negative cancer (32). Particularly, AR positivity on IHC was identified as a significant prognostic factor for the recurrence of bladder cancer (HR =0.27). Mashhadi et al. (25) provided similar findings following examination of 120 surgical specimens of UBC patients and 132 specimens from normal healthy individuals. They noted that AR positivity was significantly correlated with a higher tumor stage and grade, as well as poorer prognosis than AR negativity.
In summary, these findings strongly indicate that human bladder can be an AR target tissue and the AR signaling pathway can play an important role in bladder carcinogenesis, progression, drug resistance and oncological outcomes.
AR signaling: a novel therapeutic target in bladder cancerOther Section
- Introduction
- Expression patterns of the AR in bladder cancer
- The role of the AR signaling pathway in tumorigenesis and cancer progression in bladder cancer
- AR signaling: a novel therapeutic target in bladder cancer
- Conclusions
- Acknowledgments
- Footnote
- References
According to the number of evidences discussed in this review, AR signaling is significantly associated with bladder cancer development, progression and prognosis, and therefore, this specific pathway can be a novel therapeutic target for bladder cancer.
Notably, in vitro and in vivo bladder cancer models have demonstrated that specific inhibition of AR expression by genetic and pharmacologic agents led to induction of suppression of tumor growth and proliferation. Kawahara et al. (33) showed that dihydrotestosterone treatment enhanced the proliferation, migration and invasion capabilities of bladder cancer cells by using AR-negative and AR-positive bladder cancer models. The authors found that AR blocking agents, such as bicalutamide and enzalutamide, had significant inhibitory effects on AR signaling-mediated cancer cell proliferation and invasion, particularly in AR-positive cell lines. In a xenograft model using AR-positive bladder cancer cells, only enzalutamide reduced tumor growth among several AR antagonists. Recently, Kameyama and colleagues published an interesting paper which demonstrated that AR inhibition by siRNA treatment markedly reduced the proliferation of bladder cancer cells with intrinsic and acquired gemcitabine resistance (HTB5 and T24-GR, respectively), and enzalutamide inhibited the AR transcriptional activity and cell proliferation in these cells (34).
Furthermore, Izumi et al. (31) first provided clinical evidence which showed the relevance of the AR signaling pathway in the development of bladder cancer. They analyzed 162 prostate cancer patients with concurrent primary bladder cancer, and reported that patients treated with ADT had a lower recurrence rate of bladder cancer compared to those who were not treated with ADT for prostate cancer. More importantly, ADT was finally identified as a significant predictor of bladder cancer recurrence (HR =0.29) in these patients. These data suggest that specific inhibition of the AR signaling pathway can be a potential therapeutic strategy in patients with bladder cancer, particularly AR-positive tumors (Table 2).

Full table
Interestingly, Morales and colleagues reviewed 72,370 male individuals who participated in the Prostate, Lung, Colorectal, and Ovarian cancer screening trial, which particularly focused on the association between the use of finasteride and risk of bladder cancer (35). The authors proved that finasteride use was significantly associated with decreased risk of bladder cancer development (HR =0.63) after adjusting for age and smoking history. Recent clinical evidence suggests that 5-ARI can also reduce the risk of intravesical recurrence in patients with NMIBC (36). Of note, multivariate analysis revealed that history of androgen suppression therapy (HR =0.36) remained an independent predictor of disease recurrence, in addition to multiple lesions (HR =1.82), large size (HR =2.13) and smoking history (HR =2.45). These two studies first provided evidence that the use of 5-ARI can be a potential way to prevent the risk of cancer development and intravesical tumor recurrence following initial treatment in clinical practice (Table 2).
Currently, the research group at the University of Rochester is performing a phase II clinical trial to examine the effect of androgen blockade by administration of enzalutamide in preventing disease recurrence in patients with NMIBC (NCT02605863). Additionally, a phase I study of enzalutamide in combination with gemcitabine and cisplatin is also being conducted to determine the maximum tolerated dose of enzalutamide, as well as to assess the response rates, progression-free survival and overall survival at 6 months after initial treatment in patients with stage IV bladder cancer (NCT02300610). We anticipate that suppression of the AR signaling pathway can be a novel chemopreventive or therapeutic strategy for patients with UBC in the clinical setting.
ConclusionsOther Section
- Introduction
- Expression patterns of the AR in bladder cancer
- The role of the AR signaling pathway in tumorigenesis and cancer progression in bladder cancer
- AR signaling: a novel therapeutic target in bladder cancer
- Conclusions
- Acknowledgments
- Footnote
- References
In this review, we have summarized the accumulated evidence on involvement of AR and its target signaling pathway in cancer development, progression and oncological outcomes as well as the therapeutic potential of AR targeting agents in patients with UBC. Although the underlying molecular mechanisms involved in the role of AR in the biology of UBC are not fully understood, it seems obvious that AR targeted therapy can be a novel and promising strategy for chemoprevention and treatment of patients with UBC in the near future. For the real-world clinical application, further randomized clinical trials should be encouraged to determine the efficacy of anti-AR treatments in patients with UBC.
AcknowledgmentsOther Section
- Introduction
- Expression patterns of the AR in bladder cancer
- The role of the AR signaling pathway in tumorigenesis and cancer progression in bladder cancer
- AR signaling: a novel therapeutic target in bladder cancer
- Conclusions
- Acknowledgments
- Footnote
- References
Funding: This work was supported by a research grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (No. 2016R1A2B4011623), and by the Basic Science Research Program through the NRF funded by the Ministry of Education (No. 2016R1D1A1A02936950).
FootnoteOther Section
- Introduction
- Expression patterns of the AR in bladder cancer
- The role of the AR signaling pathway in tumorigenesis and cancer progression in bladder cancer
- AR signaling: a novel therapeutic target in bladder cancer
- Conclusions
- Acknowledgments
- Footnote
- References
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Bladder Cancer”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.04.07). The series “Bladder Cancer” was commissioned by the editorial office without any funding or sponsorship. MK served as the unpaid Guest Editors of the series. JHK served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Translational Cancer Research from Nov 2016 to Dec 2018. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Introduction
- Expression patterns of the AR in bladder cancer
- The role of the AR signaling pathway in tumorigenesis and cancer progression in bladder cancer
- AR signaling: a novel therapeutic target in bladder cancer
- Conclusions
- Acknowledgments
- Footnote
- References
- Antoni S, Ferlay J, Soerjomataram I, et al. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol 2017;71:96-108. [Crossref] [PubMed]
- Chalasani V, Chin JL, Izawa JI. Histologic variants of urothelial bladder cancer and nonurothelial histology in bladder cancer. Can Urol Assoc J 2009;3:S193-8. [Crossref] [PubMed]
- Babjuk M, Böhle A, Burger M, et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol 2017;71:447-61. [Crossref] [PubMed]
- Milowsky MI, Rumble RB, Booth CM, et al. Guideline on Muscle-Invasive and Metastatic Bladder Cancer (European Association of Urology Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J Clin Oncol 2016;34:1945-52. [Crossref] [PubMed]
- Cheetham PJ, Petrylak DP. New Agents for the Treatment of Advanced Bladder Cancer. Oncology (Williston Park) 2016;30:571-9, 588. [PubMed]
- Dobruch J, Daneshmand S, Fisch M, et al. Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes. Eur Urol 2016;69:300-10. [Crossref] [PubMed]
- Deerberg F, Rehm S, Jostmeyer HH. Spontaneous urinary bladder tumors in DA/Han rats: a feasible model of human bladder cancer. J Natl Cancer Inst 1985;75:1113-21. [PubMed]
- Okajima E, Hiramatsu T, Iriya K, et al. Effects of sex hormones on development of urinary bladder tumours in rats induced by N-butyl-N-(4-hydroxybutyl) nitrosamine. Urol Res 1975;3:73-9. [Crossref] [PubMed]
- Li Y, Izumi K, Miyamoto H. The role of the androgen receptor in the development and progression of bladder cancer. Jpn J Clin Oncol 2012;42:569-77. [Crossref] [PubMed]
- Godoy G, Gakis G, Smith CL, et al. Effects of Androgen and Estrogen Receptor Signaling Pathways on Bladder Cancer Initiation and Progression. Bladder Cancer 2016;2:127-37. [Crossref] [PubMed]
- Lombard AP, Mudryj M. The emerging role of the androgen receptor in bladder cancer. Endocr Relat Cancer 2015;22:R265-77. [Crossref] [PubMed]
- Shukla GC, Plaga AR, Shankar E, et al. Androgen receptor-related diseases: what do we know? Andrology 2016;4:366-81. [Crossref] [PubMed]
- Lonergan PE, Tindall DJ. Androgen receptor signaling in prostate cancer development and progression. J Carcinog 2011;10:20. [Crossref] [PubMed]
- Culig Z, Santer FR. Androgen receptor co-activators in the regulation of cellular events in prostate cancer. World J Urol 2012;30:297-302. [Crossref] [PubMed]
- Mikkonen L, Pihlajamaa P, Sahu B, et al. Androgen receptor and androgen-dependent gene expression in lung. Mol Cell Endocrinol 2010;317:14-24. [Crossref] [PubMed]
- Ha YS, Lee GT, Modi P, et al. Increased Expression of Androgen Receptor mRNA in Human Renal Cell Carcinoma Cells is Associated with Poor Prognosis in Patients with Localized Renal Cell Carcinoma. J Urol 2015;194:1441-8. [Crossref] [PubMed]
- Chia K, O'Brien M, Brown M, et al. Targeting the androgen receptor in breast cancer. Curr Oncol Rep 2015;17:4. [Crossref] [PubMed]
- He D, Li L, Zhu G, et al. ASC-J9 suppresses renal cell carcinoma progression by targeting an androgen receptor-dependent HIF2α/VEGF signaling pathway. Cancer Res 2014;74:4420-30. [Crossref] [PubMed]
- Williams EM, Higgins JP, Sangoi AR, et al. Androgen receptor immunohistochemistry in genitourinary neoplasms. Int Urol Nephrol 2015;47:81-5. [Crossref] [PubMed]
- Miyamoto H, Yao JL, Chaux A, et al. Expression of androgen and oestrogen receptors and its prognostic significance in urothelial neoplasm of the urinary bladder. BJU Int 2012;109:1716-26. [Crossref] [PubMed]
- Boorjian S, Ugras S, Mongan NP, et al. Androgen receptor expression is inversely correlated with pathologic tumor stage in bladder cancer. Urology 2004;64:383-8. [Crossref] [PubMed]
- Mir C, Shariat SF, van der Kwast TH, et al. Loss of androgen receptor expression is not associated with pathological stage, grade, gender or outcome in bladder cancer: a large multi-institutional study. BJU Int 2011;108:24-30. [Crossref] [PubMed]
- Kauffman EC, Robinson BD, Downes MJ, et al. Role of androgen receptor and associated lysine-demethylase coregulators, LSD1 and JMJD2A, in localized and advanced human bladder cancer. Mol Carcinog 2011;50:931-44. [Crossref] [PubMed]
- Boorjian SA, Heemers HV, Frank I, et al. Expression and significance of androgen receptor coactivators in urothelial carcinoma of the bladder. Endocr Relat Cancer 2009;16:123-37. [Crossref] [PubMed]
- Mashhadi R, Pourmand G, Kosari F, et al. Role of steroid hormone receptors in formation and progression of bladder carcinoma: a case-control study. Urol J 2014;11:1968-73. [PubMed]
- Imada S, Akaza H, Ami Y, et al. Promoting effects and mechanisms of action of androgen in bladder carcinogenesis in male rats. Eur Urol 1997;31:360-4. [PubMed]
- Miyamoto H, Yang Z, Chen YT, et al. Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst 2007;99:558-68. [Crossref] [PubMed]
- Hsu JW, Hsu I, Xu D, et al. Decreased tumorigenesis and mortality from bladder cancer in mice lacking urothelial androgen receptor. Am J Pathol 2013;182:1811-20. [Crossref] [PubMed]
- Shiota M, Takeuchi A, Yokomizo A, et al. Androgen receptor signaling regulates cell growth and vulnerability to doxorubicin in bladder cancer. J Urol 2012;188:276-86. [Crossref] [PubMed]
- Nam JK, Park SW, Lee SD, et al. Prognostic value of sex-hormone receptor expression in non-muscle-invasive bladder cancer. Yonsei Med J 2014;55:1214-21. [Crossref] [PubMed]
- Izumi K, Taguri M, Miyamoto H, et al. Androgen deprivation therapy prevents bladder cancer recurrence. Oncotarget 2014;5:12665-74. [Crossref] [PubMed]
- Izumi K, Ito Y, Miyamoto H, et al. Expression of androgen receptor in non-muscle-invasive bladder cancer predicts the preventive effect of androgen deprivation therapy on tumor recurrence. Oncotarget 2016;7:14153-60. [PubMed]
- Kawahara T, Ide H, Kashiwagi E, et al. Enzalutamide inhibits androgen receptor-positive bladder cancer cell growth. Urol Oncol 2016;34:432.e15-23. [Crossref] [PubMed]
- Kameyama K, Horie K, Mizutani K, et al. Enzalutamide inhibits proliferation of gemcitabine-resistant bladder cancer cells with increased androgen receptor expression. Int J Oncol 2017;50:75-84. [PubMed]
- Morales EE, Grill S, Svatek RS, et al. Finasteride Reduces Risk of Bladder Cancer in a Large Prospective Screening Study. Eur Urol 2016;69:407-10. [Crossref] [PubMed]
- Shiota M, Kiyoshima K, Yokomizo A, et al. Suppressed Recurrent Bladder Cancer after Androgen Suppression with Androgen Deprivation Therapy or 5alpha-Reductase Inhibitor. J Urol 2017;197:308-13. [Crossref] [PubMed]


