
The pathogenesis of follicular lymphoma, beyond apoptosis resistance
Follicular lymphoma (FL) is the second most common indolent B-cell lymphoma (1). FL is characterized by a heterogeneous clinical evolution and remains incurable despite recent progress. The accepted founding event of FL pathogenesis is the acquisition of the t(Jeny14;Jeny18) (q32;q21) during primary VDJ recombination in the bone marrow (2). This chromosomal translocation juxtaposes the anti-apoptotic proto-oncogene BCL2 to the immunoglobulin heavy chain locus during VDJ recombination (3). The resulting dysregulation of BCL2 expression provides a survival advantage through activation of antiapoptotic programs. The consequences of this early genetic event become manifest much later during B-cell maturation, when FL cells clonally expand and adopt malignant growth in germinal centers (GC) (2). The precise mechanisms involved in the initiation of malignant growth of apoptosis-resistant t(14:18)-positive B-cells remain unclear.
Secondary mutations
As a GC neoplasia, FL is characterized by the constitutive expression of activation induced cytosine deaminase (AID), an enzyme with intrinsic mutagenic capability. Continuous AID expression can result in mutation of proto-oncogenes such as BCL6, c-MYC or transcription factors as Pax-5 by aberrant somatic hypermutation (4-6). An increased susceptibility to subsequent genetic alterations may lead to acquisition of additional oncogenic events that promote lymphomagenesis and cause tumor progression (7).
Whole genome and exome sequencing studies have identified mutations that are recurrent in FL lymphomagenesis or are involved in tumor clonal evolution (8-10). The genomic landscape of FL seems to be dominated by gene mutations in epigenetic regulators, such as CREBBP, EZH2, and KMT2D. These mutations are currently proposed as early initiating events (5,8,11-13). Figure 1 depicts key molecular events in FL pathogenesis.
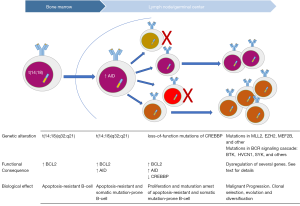
Krysiak and co-workers recently reported on recurrent mutations identified in a cohort of 113 FL cases determined by sequencing of a custom capture panel of 1,716 genes (14). The custom capture panel design was based on exome sequencing data of 24 FL patients and included genes recurrently mutated in other mature B-cell lymphomas (14). In contrast to most prior studies, the cohort reported by Krysiak et al. consisted primarily of asymptomatic, low-risk, treatment-naive FL patients. In earlier studies, recurrent mutations have been reported in the histone methyltransferases MLL2 (89%) and EZH2 (7.2%), the histone acetylases CREBBP (32.6%), EP300 (8.7%), and MEF2B (15.3%) (2). Krysiak et al. describe a higher overall number and rate of histone gene mutations than previously observed: KMT2D/MLL2 (60.0%), CREBBP (52.4%), EP300 (19.0%), EZH2 (17.0%), MEF2B (7.6%). This study also identified frequent co-occurrence of these mutations within individual patients.
Current evidence indicates that activating mutations of the histone methyltransferase EZH2 contribute to the FL cells arrest at the GC stage (11,13). More recently, it has been reported that inactivating mutations of the global transcriptional coactivator CREBBP may facilitate malignant transformation by conferring proliferative advantages and interfering with terminal differentiation of B-cells (15).
The high recurrence of these mutations illustrates the role of epigenetic changes in FL pathogenesis and also draws attention to the role of AID-dependent DNA demethylation in lymphoid neoplasms (16). Putative epigenetic changes induced by constitutive AID expression deserves further investigation (17).
Krysiak et al. also report on the overrepresentation of TP53 and EP300 in transformed FL, and of HIST1H3G in posttreatment samples as compared with treatment-naive cases. Despite the limited statistical power of the study, these findings suggest a role of TP53 and EP300 mutations in FL progression and warrants further investigation.
The role of the B-cell receptor
An interesting finding in Krysiak’s work is the detection of recurrent somatic mutations affecting the B-cell receptor (BCR) signaling pathway genes (14). These results should be discussed together with our current understanding on the role of the BCR in FL biology.
Earlier evidence depicts unique characteristics of the FL BCR such as high hypermutation rates, distinctive selection patterns, mannosylation of the antigen binding site and autoantigen binding (18-21). In addition, a biological therapy selectively targeting the BCR can induce clinical responses, even when used as upfront monotherapy (22). Altogether, this evidence suggests that FL cell survival relies on a highly selected and functional BCR. On the other hand, the Bruton's tyrosine kinase (BTK) inhibitor ibrutinib, which targets an essential component of the BCR signaling cascade, appears less active in FL than in MCL and CLL (23).
Krysiak et al. described significantly mutated genes in the interconnected BCR and CXCR4 signaling pathways in 45% of FL patients (14). In addition to previously reported genes, such as CD79B, CARD11, CXCR4, and SYK, they describe novel mutations in BTK and HVCN1. In particular, mutations found in genes acting downstream the BCR signaling cascade may provide a rationale for the less robust effect of ibrutinib in FL than observed in other mature B-cell malignancies (23,24).
Recently, Compagno et al. report on the increase of AID-dependent genomic instability in normal and neoplastic B cells after ibrutinib and idelalisib treatment (25). This interesting work ponders about the use of BTK inhibitors in lymphoid neoplasms and highlights the importance of DNA damage induced by new therapies aimed to abrogate BCR signaling.
In this scenario, the understanding of the precise interplay between the tumor dependence on a functional BCR and the presence of recurrent mutations in the BCR signaling cascade remains elusive and warrants future functional studies.
Conclusions and perspective
The diagnostic category of FL is heterogeneous in terms of morphology, genetics, and biologic behavior. The understanding of FL pathogenesis remains essential for the development of novel therapeutic strategies for this incurable disease.
Next generation sequencing studies, such as the work by Fehniger’s group, are helping to depict the complex genetic profile of FL. This heterogeneous genomic landscape seems to overlay a common molecular backbone characterized by overexpression of BCL2, constitutive AID expression and mutations in epigenetic regulators.
Further research is needed for the determination of the clinical and biological relevance of these novel findings. The ongoing determination of “targetable” genetic aberrations is currently pushing forward the development of molecularly driven targeted therapeutics. In FL, the intrinsic heterogeneity and the evolving tumor dynamics add an extra layer of complexity. Successful targeted therapy will require a profound understanding of not only the FL genetic profile, but the forces driving the evolving tumor dynamics and the forces driving the (sub)clonal selection process.
Acknowledgments
The authors appreciate the critical review of Prof. Dr. H. Veelken, Department of Hematology, Leiden University Medical Center.
Funding: M.A.N. is supported by FONDECYT [11140542] and by the European Union 7th Framework Program [PIRSES-GA-2013-612583].
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Peipei Xu (Department of Hematology, The Affiliated Drum Tower Hospital of Nanjing University Medical School, Nanjing, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.05.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood 2006;107:265-76. [Crossref] [PubMed]
- Kridel R, Sehn LH, Gascoyne RD. Pathogenesis of follicular lymphoma. J Clin Invest 2012;122:3424-31. [Crossref] [PubMed]
- Tsujimoto Y, Gorham J, Cossman J, et al. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science 1985;229:1390-3. [Crossref] [PubMed]
- Liu M, Duke JL, Richter DJ, et al. Two levels of protection for the B cell genome during somatic hypermutation. Nature 2008;451:841-5. [Crossref] [PubMed]
- Scherer F, Navarrete MA, Bertinetti-Lapatki C, et al. Isotype-switched follicular lymphoma displays dissociation between activation-induced cytidine deaminase expression and somatic hypermutation. Leuk Lymphoma 2016;57:151-60. [Crossref] [PubMed]
- Reiniger L, Bödör C, Bognár A, et al. Richter's and prolymphocytic transformation of chronic lymphocytic leukemia are associated with high mRNA expression of activation-induced cytidine deaminase and aberrant somatic hypermutation. Leukemia 2006;20:1089-95. [Crossref] [PubMed]
- Bende RJ, Smit LA, Van Noesel CJ. Molecular pathways in follicular lymphoma. Leukemia 2007;21:18-29. [Crossref] [PubMed]
- Okosun J, Bödör C, Wang J, et al. Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nat Genet 2014;46:176-81. [Crossref] [PubMed]
- Morin RD, Mendez-Lago M, Mungall AJ, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature 2011;476:298-303. [Crossref] [PubMed]
- Green MR, Gentles AJ, Nair RV, et al. Hierarchy in somatic mutations arising during genomic evolution and progression of follicular lymphoma. Blood 2013;121:1604-11. [Crossref] [PubMed]
- Béguelin W, Popovic R, Teater M, et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell 2013;23:677-92. [Crossref] [PubMed]
- Okosun J, Montoto S, Fitzgibbon J. The routes for transformation of follicular lymphoma. Curr Opin Hematol 2016;23:385-91. [Crossref] [PubMed]
- Caganova M, Carrisi C, Varano G, et al. Germinal center dysregulation by histone methyltransferase EZH2 promotes lymphomagenesis. J Clin Invest 2013;123:5009-22. [Crossref] [PubMed]
- Krysiak K, Gomez F, White BS, et al. Recurrent somatic mutations affecting B-cell receptor signaling pathway genes in follicular lymphoma. Blood 2017;129:473-83. [Crossref] [PubMed]
- Zhang J, Vlasevska S, Wells VA, et al. The CREBBP acetyltransferase is a haploinsufficient tumor suppressor in b-cell lymphoma. Cancer Discov 2017;7:322-37. [Crossref] [PubMed]
- Bhutani N, Brady JJ, Damian M, et al. Reprogramming towards pluripotency requires AID-dependent DNA demethylation. Nature 2010;463:1042-7. [Crossref] [PubMed]
- Kumar R, Dimenna L, Schrode N, et al. AID stabilizes stem-cell phenotype by removing epigenetic memory of pluripotency genes. Nature 2013;500:89-92. [Crossref] [PubMed]
- Sachen KL, Strohman MJ, Singletary J, et al. Self-antigen recognition by follicular lymphoma B-cell receptors. Blood 2012;120:4182-90. [Crossref] [PubMed]
- Coelho V, Krysov S, Ghaemmaghami AM, et al. Glycosylation of surface Ig creates a functional bridge between human follicular lymphoma and microenvironmental lectins. Proc Natl Acad Sci U S A 2010;107:18587-92. [Crossref] [PubMed]
- Scherer F, van der Burgt M, Kielbasa SM, et al. Selection patterns of B-cell receptors and the natural history of follicular lymphoma. Br J Haematol 2016;175:972-5. [Crossref] [PubMed]
- Papaioannou D, Strothmeyer AM, Dühren-Von Minden M, et al. Evidence for idiotype-directed immunosurveillance is restricted to follicular lymphoma and attributable to somatic hypermutation. Haematologica 2015;100:e143-6. [Crossref] [PubMed]
- Navarrete MA, Heining-Mikesch K, Schüler F, et al. Upfront immunization with autologous recombinant idiotype Fab fragment without prior cytoreduction in indolent B-cell lymphoma. Blood 2011;117:1483-91. [Crossref] [PubMed]
- Bartlett NL, LaPlant BR, Qi J, et al. Ibrutinib Monotherapy in Relapsed/Refractory Follicular Lymphoma (FL): Preliminary Results of a Phase 2 Consortium (P2C) Trial. Blood 2014;124:800.
- Ujjani CS, Jung SH, Pitcher B, et al. Phase 1 trial of rituximab, lenalidomide, and ibrutinib in previously untreated follicular lymphoma: Alliance A051103. Blood 2016;128:2510-6. [Crossref] [PubMed]
- Compagno M, Wang Q, Pighi C, et al. Phosphatidylinositol 3-kinase δ blockade increases genomic instability in B cells. Nature 2017;542:489-93. [Crossref] [PubMed]


