
Emerging targeted therapies in advanced bladder cancer
Introduction
Urothelial bladder cancer (UBC) is the ninth most common cancer worldwide, as over 300,000 people are diagnosed every year, with an annual death of at least 120,000 people (1). Approximately 70% of bladder cancers are of the non-muscle invasive type, which has a favorable prognosis of 85% 5-year survival rate. However, the other 30% are invasive or metastatic, which have poor prognoses and a high tendency of recurrence and distant metastasis (2-4). Currently, the standard therapy for recurrent or metastatic UBC is platinum-based chemotherapy (4,5). However, the clinical outcome of standard chemotherapy is disappointing, with the 5-year survival rate being only approximately 10% (6). Moreover, only approximately 40% of patients respond to platinum-based chemotherapy, and for those who do not respond or have progressed after chemotherapy, the median survival time is only 9 months (7). As such, a novel approach is necessary to overcome this therapeutic challenge in treating advanced UBC. Moreover, as the median age of diagnosis of UBC is 65 years, presence of comorbidities, such as renal impairment, makes more than a third of advanced UBC patients ineligible for the standard cytotoxic chemotherapy (8,9). In order to resolve these limitations, various molecular targeting agents are currently being investigated. The most notable have been the immune checkpoint inhibitors (ICI), which include the anti-PD-L1 antibody, atezolizumab, that has been approved by the Food and Drug Administration (FDA) as a second-line therapy for UBC (10,11). This achievement has dramatically advanced the treatment outcome of advanced UBC. This review paper aims to provide insights on the currently available and promising systemic targeted therapies in UBC.
ICI
Recently, immunotherapy has been emerging as a potent treatment for various solid tumors, and several immunotherapeutic drugs have already been approved by the FDA (12). In UBC, clinical trials have demonstrated that ICIs like atezolizumab have durable anti-cancer efficacy and survival benefits. ICI is a treatment that blocks immune-regulatory proteins expressed in immune cells or tumor cells. Among them, the most critical immune-checkpoints are PD-1/PD-L1 and CTLA-4/B7. The PD-1/PD-L1 pathway restricts T cell effector function in the peripheral tumor microenvironment, while the CTLA-4/B7 pathway suppresses T cell activation and expansion in central lymphoid organs (13). Furthermore, various studies have shown that PD-L1 overexpression is correlated with a poor prognosis (14). Such results can be attributed to the tumors’ ability to evade anti-cancer immune responses by increased PD-1/PD-L1 expression and via the CTLA-4/B7 pathway. PD-1/PD-L1 and CTLA-4 inhibitors can block these specific targets, making them effective molecular targeted cancer therapies for UBC. In addition, studies have proven that patients with high PD-L1 expression show high overall response rates (ORR) to ICI (15). Although not yet fully elucidated, PD-L1 is now being considered as a potential biomarker for prognosis, and response to ICI (12). Consequently, in UBC, it is necessary to understand the efficacy and survival benefits of various ICIs and to determine whether PD-L1 overexpression is a potential predictive biomarker for ICIs (Table 1).
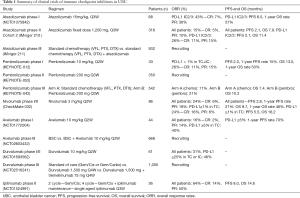
Full table
Atezolizumab
Atezolizumab is a fully humanized monoclonal IgG1 antibody against PD-L1 that inhibits the interactions of PD-L1 with PD-1 and B7.1 (16). Atezolizumab was the first drug to be approved as salvage therapy for advanced UBC by the FDA (11). In a phase I study performed on patients with locally advanced or metastatic solid tumors, atezolizumab was administered at a dose of 15 mg/kg every 3 weeks. Among the 68 UBC patients enrolled in the study, 67 were evaluable for efficacy, which was analyzed according to PD-L1 expression status in tumor-infiltrating immune cells. PD-L1 positive was defined as immunohistochemistry (IHC) score 2 or 3 (2/3), while PD-L1 negative was defined as IHC score 0 or 1 (0/1), with the IHC score representing PD-L1 expression levels in tumor-infiltrating immune cells (IHC 0 ≤1%, IHC 1 =1–5%, IHC 2 =5–10%, and IHC 3 ≥10%). The PD-L1 positive group showed a response rate of 43%, whereas the PD-L1 negative group showed a response rate of 11% (17). Altogether, the ORR of the entire efficacy-evaluable UBC patients was 26%. Following these findings, an updated survival data for atezolizumab therapy was presented at the 2015 American Society of Clinical oncology meeting (18). In the PD-L1 positive group, ORR was 46% and median progression-free survival (PFS) was 6 months with a 1-year overall survival (OS) rate of 57%. The median duration of response (mDOR) and median OS were not reached for the efficacy-evaluable patients in the study. Atezolizumab was well tolerated in most patients; 64% of patients had all-grade treatment-related adverse event (AE), while a grade 3–4 AE occurred in only 8% (18). These results led to a phase II study (IMvigor210) consisting of two cohort trials. Cohort 1 consisted of cisplatin-ineligible patients who were chemotherapy-naive. Cohort 2 consisted of patients who had progressed after prior platinum-based therapy. A fixed dose of 1200 mg atezolizumab was administered every 3 weeks. In cohort 2, the ORR of the all-patient group (n=310) was 15%, with an ORR of 26% in the PD-L1 positive group. This indicates that higher PD-L1 expression in immune cells could predict a better response to atezolizumab treatment. The median PFS was 2.1 months in both groups (all-patients and PD-L1 positive), while the median OSs were 7.9 months for all-patients and 11.4 months for the PD-L1 positive group (19). Following these results, a primary analysis of cohort 1 was also reported recently. In the all-patient group (n=119), the ORR and PFS were 19% and 2.1 months, respectively. Of the 23 responses, 22 were ongoing with mDOR not yet reached. In the PD-L1 positive group, the ORR and PFS were 22% and 2.9 months, respectively. The median OS was 10.6 months regardless of PD-L1 expression. Regarding the safety profile, the rate of grade 3–4 treatment-related AEs was 12%, and the rate of grade 3–4 immune-mediated AEs was 3%. The most common AEs were fatigue, pruritus, and diarrhea (10). These results demonstrate that atezolizumab has durable activity and good tolerance in advanced UBC. A phase III study (IMvigor211) consisting of 932 patients with locally advanced or metastatic urothelial cancer (UC), is currently ongoing. This study seeks to compare chemotherapy (vinflunine, paclitaxel, or docetaxel) with and without atezolizumab.
Pembrolizumab
Pembrolizumab is a humanized monoclonal IgG4 antibody against PD-1 that blocks PD-1 interaction with both PD-L1 and PD-L2 (20). Pembrolizumab showed anti-tumor efficacy in several solid tumors, and consequently, was approved by the FDA for advanced melanoma, advanced non-small cell lung cancer (NSCLC), and head and neck cancer (21). A phase I study (KEYNOTE-012) indicated that pembrolizumab has durable anti-tumor activity and a tolerable safety profile in advanced UC (22,23). Thirty-three patients with PD-L1 positive recurrent or metastatic UC, who previously failed platinum-based chemotherapy, were assigned to receive 10 mg/kg of pembrolizumab every 2 weeks. PD-L1 positivity was defined as a PD-L1 expression of greater than 1% in tumor cells or immune cells (TC + IC). The ORR was 26%. The PFS and 1-year PFS rates were 2 months and 15%, respectively. The median OS was 13 months with the 1-year OS rate being 50%. In addition, pembrolizumab showed acceptable safety with the rate of grade 3–4 treatment-related AEs being 15%. Four deaths occurred during the study, however, they were not treatment-related (23). A phase II study (KEYNOTE-052) is currently ongoing. In this study, 200 mg of pembrolizumab is administered every 3 weeks to unresectable or metastatic UC patients ineligible to receive cisplatin (24). A randomized phase III clinical trial (KEYNOTE-045) was performed to compare pembrolizumab with chemotherapy in UC patients who recurred or progressed following platinum-based chemotherapy (25). Patients were randomly allocated in a 1:1 ratio to receive pembrolizumab (200 mg fixed dose) or investigator’s choice of chemotherapy, every 3 weeks. In the pembrolizumab group, the median OS and 1-year OS rate were 10.3 months and 43.9%, respectively. These were significantly higher than those of chemotherapy group (OS: 7.4 months, 1-year OS rate: 30.7%). Regarding PFS, there was no significant difference between the two groups (2.1 vs. 3.3 months, P=0.42). The ORR was 21.1% in the pembrolizumab group, as compared with 11.4% in the chemotherapy group. In a safety comparison, treatment-related AEs were remarkably lower in the pembrolizumab group compared to the chemotherapy group. Accordingly, these results raise anticipation for the acceptance of pembrolizumab as a novel second-line therapy for recurrent or metastatic UBC.
Nivolumab
Nivolumab is a fully human monoclonal IgG4 antibody against PD-1 that was approved by the FDA for NSCLC, melanoma, renal cell carcinoma and Hodgkin lymphoma (26). In a phase I/II study, nivolumab was administered every 2 weeks at 3 mg/kg to patients with metastatic UC (27). The ORR was 24.4%, median OS was 9.7 months, and the 1-year OS rate was 46%. Median PFS was 2.8 months and the 1-year PFS rate was 21%. Positive PD-L1 expression was defined as ≥1% staining of tumor cell membranes. In the PD-L1 positive subgroup, the ORR was 24%. The median OS was 16.2 months and the median PFS was 5.5 months, both of which were higher than in the all-patient group. Nivolumab was well tolerated with the rate of grade 3–4 treatment-related AE being 22%, with the most common AEs being elevated lipase, elevated amylase, and fatigue. Two patients discontinued treatment due to grade 4 pneumonitis and grade 4 thrombocytopenia (28).
Avelumab
Avelumab is a human IgG1 monoclonal antibody against PD-L1 (29). Patients with pre-treated platinum-refractory or cisplatin-ineligible UBC received treatment with avelumab at 10 mg/kg every 2 weeks in a phase I study (30). In total 44 patients who were treated with avelumab, and ORR was 15.9%. In the PD-L1 positive subgroup, in which tumor cell PD-L1 staining was ≥5%, ORR was 40%. These results suggest that higher PD-L1 expression is correlated with a better response to avelumab. The rate of any-grade treatment-related AE was 59.1% (26 patients), with only one grade 3 event, and no treatment-related death (31). Based on this result, a phase III study is ongoing to compare the best supportive care, with and without avelumab.
Durvalumab
Durvalumab is a human IgG1 monoclonal antibody against PD-L1 with potential immune checkpoint inhibitory- and anti-neoplastic activities (32). A phase I/II study was performed on patients with locally advanced or metastatic UC, who were administered durvalumab at 10 mg/kg every 2 weeks. The ORR was 31% in the all-patient group, and 46.4% in the PD-L1 positive subgroup; the PD-L1 positive subgroup was defined as ≥25% of tumor cells or ≥25% of immune cells expressing PD-L1. In contrast, the ORR within the PD-L1 negative subgroup was 0%. The rate of any-grade treatment-related AEs was 63.9%, but the rate of grade 3 AEs was only 5% (33). These results show that durvalumab is a potent and safe treatment with significant clinical efficacy in UBC patients. Based on these findings, a phase III study comparing the therapeutic effects of standard of care (gemcitabine with cisplatin or carboplatin), durvalumab, and durvalumab in combination with tremelimumab, is currently ongoing in patients with unresectable stage IV UBC.
Ipilimumab
Ipilimumab is a recombinant human IgG1 monoclonal antibody directed against human CTLA-4, that blocks the interaction of CTLA-4 with both B7.1 and B7.2 (34). In a phase II study, gemcitabine with cisplatin (GC) was administered for the first two cycles, then for the next four cycles, GC was combined with ipilimumab, in patients with chemo-naive unresectable or metastatic UC (23). For those who did not exhibit AEs, single-agent ipilimumab was administered every 3 weeks as additional maintenance. The ORR was 64% and the median PFS was 8 months. The most common grade 3–4 immune-related AEs were colitis (6%), hypophysitis (3%), hyperthyroidism (1%), and rash (1%) (35).
VEGF/R targeted therapy
Angiogenesis is a promising therapeutic target for anti-tumor therapy that has been validated in many solid tumors, such as colorectal, gastric, kidney, and lung cancer (36,37). However, no such validation has yet been reported for UBC. The two major targets of anti-angiogenic treatments are vascular endothelial growth factor/receptor (VEGF/R) and fibroblast growth factor/receptor (FGF/R). Both shall be discussed further, below (see also Table 2).

Full table
Bevacizumab
Bevacizumab, a humanized monoclonal antibody against VEGF-A, is a promising combination partner to doublet GC therapy in UBC (38). In a single-arm phase II study of bevacizumab in addition to GC, the ORR and OS were 72.0% and 19.1 months (38), respectively (6). Similar, though non-significant, results were seen when bevacizumab was combined with gemcitabine and carboplatin therapy for cisplatin-ineligible patients in another phase II study (39). This GC combination therapy with bevacizumab, however, resulted in grade 3–4 deep venous thrombosis or pulmonary embolism (DVT/PE), which was observed in 21% of patients. The high incidence was most likely due to the initial gemcitabine dosage of 1,250 mg/m2, which was thereafter reduced to 1,000 mg/m2 for the remainder of the study. This reduced the occurrence of grade 3–4 DVT/PE from 39% to 8%. Despite the AEs, the clinically significant advantages of ORR and OS paved the way for a randomized placebo-controlled phase III study, which has finished accrual, with results soon to be presented.
Ramucirumab
The positive results of combining bevacizumab with GC in metastatic UC patients have led to the testing of other anti-angiogenic agents. One worth noting is ramucirumab, a fully human monoclonal antibody that, unlike bevacizumab, binds to a receptor (VEGFR-2) instead of a ligand (40). Ramucirumab had demonstrated efficacy and a favorable toxicity profile in gastro-esophageal cancer patients and has been approved for use in combination with docetaxel for the treatment of metastatic NSCLC that progressed during or after platinum-based therapy (41,42). In a randomized phase II study in patients with locally advanced or metastatic UC, combining docetaxel with ramucirumab resulted in a statistically significant, superior PFS of 5.4 months. This is nearly twice that of the docetaxel-alone arm (2.8 months; P=0.0002) (43). The OS, by contrast, was not significantly different between the two arms (10.4 vs. 9.2 months, P=0.201). Therapy-related grade 3–4 AEs were more frequent in the combination arm, with the most common events being fatigue (30%) and anemia (13%). Nevertheless, the significant benefit of PFS was enough to initiate a randomized, double-blind, placebo-controlled phase III study (RANGE trial) in order to confirm the efficacy of ramucirumab in UBC patients. This study is currently ongoing.
Feasibility of combining vascular targeting therapy with ICI
Recently, VEGF/R-targeted therapy in combination with ICI is an emerging therapy that deserves attention for the following two rationales based on preclinical studies (44). Firstly, blockade of the VEGF pathway in UBC augments dendritic cell maturation, which in turn enhances T cell activation in lymphoid organs (45). Secondly, targeting the VEGF pathway induces vascular normalization of malformed and malfunctioning tumor vasculatures, resulting in better intratumoral T cell infiltration, and thus, more effective killing of tumor cells by tumor-specific T cells (46). Together, these findings have led to various clinical trials combining VEGF/R inhibitors with checkpoint immunotherapy in different tumor types. One notable phase I study examined the combination of atezolizumab and bevacizumab in the treatment of metastatic renal cell carcinoma. In this study, PR was achieved in 40% of patients, with T cell infiltration being markedly enhanced in the tumor tissues (47,48). Similar results were reported in a malignant melanoma study that combined ipilimumab with bevacizumab. The combination of atezolizumab and bevacizumab is also currently being studied in a phase II study, in a first line context for cisplatin-ineligible patients with metastatic UC (49).
FGFR targeted therapy
Considering that nearly 40% of UBC patients possess genetic alterations in the FGF/R gene, the FGF/R pathway seems to be a promising therapeutic target for metastatic UC patients with FGF/R genetic alterations (50).
JNJ42756493
JNJ42756493, a potent pan-FGFR inhibitor, has been receiving attention due to a high response rate in metastatic UC with FGFR pathway alteration. In a phase I study that enrolled 65 patients with advanced solid tumor, irrespective of FGFR alteration, 8 were UBC patients who had failed at least four previous lines of treatment (51). Among the 23 response-evaluable patients (with FGFR1–4 or FGF3/FGF4 alterations), PR was achieved by 4 patients, including 3 of the UBC patients (37.5%), suggesting high efficacy of JNJ42756493 in UBC. In this study, the most common treatment-related AEs included hyperphosphatemia, asthenia, and dry mouth. There were no treatment-related deaths. These findings led to a phase II study of JNJ42756493 in metastatic or surgically unresectable UC patients with FGFR genomic alterations, which is currently accruing (52).
BGJ398
Another potential FGFR-targeting agent is BGJ398, an orally bioavailable FGFR1–3 tyrosine kinase inhibitor. In a phase I study that accrued 132 advanced solid tumor patients with FGFR alterations, 5 out of 8 (62.5%) UC patients carrying an FGFR3 alteration experienced tumor reduction, with 3 (37.5%) achieving PR (53). The AEs were mostly grade 1–2 and manageable. To further evaluate the efficacy of BGJ398 in UC patients harboring FGFR3 mutation or fusion, a fourth expansion arm was added and is currently accruing (54).
EGFR/HER2 targeted therapy
The epidermal growth factor receptor (EGFR) family of tyrosine kinases, including human epidermal growth factor receptor 2 (HER2), play crucial roles in regulating cell proliferation, differentiation, migration, and apoptosis. The EGFR family of receptors is highly expressed in UBC. Dysregulation of these receptors is frequently involved in progression and metastasis of UBC. In this section, EGFR-targeted agents and HER2- targeted agents will be summarized (Table 3).

Full table
Gefitinib
Gefitinib, an oral selective EGFR tyrosine kinase inhibitor, was studied in UBC. In a single-arm phase II study (CALGB 90102), 58 patients were enrolled to received 6 cycles of chemotherapy together with GC plus gefitinib. Maintenance gefitinib was continued for responding or stable disease. Among 54 response-evaluable patients, the ORR was 42.6% with the median PFS and OS being 7.4 and 15.1 months, respectively. In terms of toxicity, this combination was generally well-tolerated. Grade 3–4 hematologic toxicity was observed in 24 patients (42%), whereas 43 patients (80%) showed grade 3–4 non-hematologic toxicity. The most common grade 3–4 non-hematological toxicity included skin-rash (20%) and diarrhea (28%). Thus the triplet combination of GC plus gefitinib has acceptable toxicity and a positive response in metastatic UBC (55).
Cetuximab
Cetuximab, a chimeric monoclonal antibody against EGFR, was also analyzed in UBC. In a randomized phase II study, 88 patients with advanced UC were allocated at a ratio of 1:2 to GC or to GC plus cetuximab. The ORR was 57.1% for the GC arm and 61.4% for the GC plus cetuximab arm. The median PFS was 8.5 months in the GC group and 7.6 months in the GC plus cetuximab group, and the median OS was 17.4 months in the GC group and 14.3 months in the GC plus cetuximab group. With regard to toxicity, both arms showed similar AEs, such as myelosuppression and nausea. However, grade 3–4 acneiform rash (25%), hypersensitivity reactions (5%), and hypomagnesemia (12%) were observed in the GC plus cetuximab group, whereas no patients showed any of these AEs in the GC group (56). As illustrated here, the GC plus cetuximab combination does not increase survival and has more AEs than the standard GC therapy. Thus, it was concluded that cetuximab is not a reasonable add-on for UBC patients.
Trastuzumab
Trastuzumab is a humanized monoclonal antibody binding to HER2. In an earlier single-arm phase II study, 44 advanced UC patients with HER2 overexpression were treated with TPCG (trastuzumab, paclitaxel, carboplatin, and gemcitabine) as the first-line therapy (57). The treatment included a 4 mg/kg loading dose of trastuzumab followed by 2 mg/kg of trastuzumab on days 1, 8, and 15. The ORR was an impressive 70% with the median PFS being 9.3 months and the median OS, 14.1 months. However, toxicity of a grade ≥3–4 was found in 98% of patients, with the most common grade 3–4 toxicities being myelosuppression (95%), neuropathy (14%), and cardiac toxicity (14%). One patient had LV dysfunction and another had sinus tachycardia. Furthermore, 2 patients treated with TPCG died after treatment due to infectious complications. In short, TPCG therapy is outstanding in terms of response, but toxic due to AEs.
Lapatinib
Lapatinib, an oral tyrosine kinase inhibitor targeting both EGFR and HER2, was expected to suppress EGFR-related UBC. In a phase I study, 17 advanced UBC patients were treated with 750, 1,000 or 1,250 mg lapatinib in a 3+3 protocol. The therapy consisted of GC plus lapatinib every 28 days. The ORR was 58.8% with a median OS of 15 months. Toxicities included leukopenia, thrombocytopenia, fatigue, arthralgia, myalgia, nausea, vomiting, and alopecia. The most common grade 3–4 hematological AEs were neutropenia (70%), thrombocytopenia (41%), and anemia (11%). The most common non-hematological grade 3–4 toxicities included nausea (6%), and renal (12%) and pulmonary (6%) AEs (58). Another phase II/III trial evaluated 232 EGFR/HER2 positive patients among 455 screened patients. The patients were treated with either a maintenance therapy of lapatinib (n=116) or a placebo (n=116) upon completion of standard chemotherapy. The median number of previous chemotherapy cycles was 6, and 64.1% of the patients had previously received cisplatin-based chemotherapy. The ORR of the lapatinib group was 13.8%, compared to 7.8% in the placebo group. The median PFS was 4.6 months for the lapatinib group and 5.3 months for the placebo group, while the median OS was 12.6 months for the lapatinib group and 11.9 months for the placebo group. The rates of grade 3–4 toxicities were 24.3% for the lapatinib group and 15.5% for the placebo group. Although survival and response increased, lapatinib did not show a significant increase in efficacy compared to the historical control (59).
Conclusions
Drug development for advanced UBC has been lagging behind that of other malignancies. Fortunately, versatile treatments have been introduced recently, including ICI, VEGF/R, FGF/R, EGFR, and HER2-targeted therapies. Among them, ICIs, such as atezolimumab, have shown the most promising outcomes. Atezolimumab has already been approved by the FDA as a standard, second-line therapy regimen, and a phase III study is now ongoing to validate its efficacy as a first-line therapy. As outlined here, the efficacy of monotherapy for UBC has been shown in several clinical trials. Now is the time to innovate possible combination therapies for optimal UBC treatment, using the results of monotherapy as the necessary stepping stones.
Acknowledgments
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science, ICT & Future Planning) (NRF-2016R1C1B2014671).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ja Hyeon Ku, Kunyoo Shin and Minyong Kang) for the series “Bladder Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.05.43). The series “Bladder Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Antoni S, Ferlay J, Soerjomataram I, et al. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol 2017;71:96-108. [Crossref] [PubMed]
- Catalona WJ, Hudson MA, Gillen DP, et al. Risks and benefits of repeated courses of intravesical bacillus Calmette-Guerin therapy for superficial bladder cancer. J Urol 1987;137:220-4. [PubMed]
- Cheung G, Sahai A, Billia M, et al. Recent advances in the diagnosis and treatment of bladder cancer. BMC Med 2013;11:13. [Crossref] [PubMed]
- Aragon-Ching JB, Trump DL. Systemic therapy in muscle-invasive and metastatic bladder cancer: current trends and future promises. Future Oncol 2016;12:2049-58. [Crossref] [PubMed]
- Cimino GD, Pan C-x, Henderson PT. Personalized medicine for targeted and platinum-based chemotherapy of lung and bladder cancer. Bioanalysis 2013;5:369-91. [Crossref] [PubMed]
- von der Maase H, Hansen S, Roberts J, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000;18:3068-77. [Crossref] [PubMed]
- Oing C, Rink M, Oechsle K, et al. Second Line Chemotherapy for Advanced and Metastatic Urothelial Carcinoma: Vinflunine and Beyond—A Comprehensive Review of the Current Literature. The J Urol 2016;195:254-63. [Crossref] [PubMed]
- Shah JB, McConkey DJ, Dinney CP. New strategies in muscle-invasive bladder cancer: on the road to personalized medicine. Clin Cancer Res 2011;17:2608-12. [Crossref] [PubMed]
- Dash A, Galsky MD, Vickers AJ, et al. Impact of renal impairment on eligibility for adjuvant cisplatin—based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer 2006;107:506-13. [Crossref] [PubMed]
- Balar AV, Galsky MD, Loriot Y, et al. Atezolizumab (atezo) as first-line (1L) therapy in cisplatin-ineligible locally advanced/metastatic urothelial carcinoma (mUC): primary analysis of IMvigor210 cohort 1. J Clin Oncol 2016;34.
- Sonpavde G. PD-1 and PD-L1 Inhibitors as Salvage Therapy for Urothelial Carcinoma. N Engl J Med 2017;376:1073-4. [Crossref] [PubMed]
- Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med 2016;14:73. [Crossref] [PubMed]
- Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol 2016;39:98-106. [Crossref] [PubMed]
- Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther 2015;14:847-56. [Crossref] [PubMed]
- Nakanishi J, Wada Y, Matsumoto K, et al. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother 2007;56:1173-82. [Crossref] [PubMed]
- Inman BA, Longo TA, Ramalingam S, et al. Atezolizumab: A PD-L1-Blocking Antibody for Bladder Cancer. Clin Cancer Res 2017;23:1886-90. [Crossref] [PubMed]
- Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558-62. [Crossref] [PubMed]
- Petrylak DP, Powles T, Bellmunt J, et al. A phase Ia study of MPDL3280A (anti-PDL1): Updated response and survival data in urothelial bladder cancer (UBC). J Clin Oncol 2015;33:abstr 4501.
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909-20. [Crossref] [PubMed]
- Tan S, Zhang CW, Gao GF. Seeing is believing: anti-PD-1/PD-L1 monoclonal antibodies in action for checkpoint blockade tumor immunotherapy. Signal Transd Targeted Ther 2016;1:e16029 [Crossref]
- Khoja L, Butler MO, Kang SP, et al. Pembrolizumab. J Immunother Cancer 2015;3:36. [Crossref] [PubMed]
- Plimack ER, Bellmunt J, Gupta S, et al. Pembrolizumab (MK-3475) for advanced urothelial cancer: Updated results and biomarker analysis from KEYNOTE-012. J Clin Oncol 2015;33:abstr 4502.
- Plimack ER, Bellmunt J, Gupta S, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol 2017;18:212-20. [Crossref] [PubMed]
- Bajorin DF, Plimack ER, Siefker-Radtke AO, et al. KEYNOTE-052: Phase 2 study of pembrolizumab (MK-3475) as first-line therapy for patients (pts) with unresectable or metastatic urothelial cancer ineligible for cisplatin-based therapy. J Clin Oncol 2015;33:abstr TPS4572.
- Bellmunt J, Sonpavde G, De Wit R, et al. editors. KEYNOTE-045: randomized phase 3 trial of pembrolizumab (MK-3475) versus paclitaxel, docetaxel, or vinflunine for previously treated metastatic urothelial cancer. ASCO Meeting Abstracts, 2015.
- Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer 2015;112:1421-7. [Crossref] [PubMed]
- Shao Z, Wang AZ, George DJ, et al. Novel immunotherapy approaches for metastatic urothelial and renal cell carcinoma. Asian J Urol 2016;3:268-77. [Crossref]
- Sharma P, Callahan MK, Bono P, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol 2016;17:1590-8. [Crossref] [PubMed]
- Boyerinas B, Jochems C, Fantini M, et al. Antibody-Dependent Cellular Cytotoxicity Activity of a Novel Anti-PD-L1 Antibody Avelumab (MSB0010718C) on Human Tumor Cells. Cancer Immunol Res 2015;3:1148-57. [Crossref] [PubMed]
- Tsiatas M, Grivas P. Immunobiology and immunotherapy in genitourinary malignancies. Ann Transl Med 2016;4:270. [Crossref] [PubMed]
- Apolo AB, Infante JR, Hamid O, et al. Safety, clinical activity, and PD-L1 expression of avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with metastatic urothelial carcinoma from the JAVELIN Solid Tumor phase Ib trial. J Clin Oncol 2016;34:abstr 367.
- Gaillard SL, Secord AA, Monk B. The role of immune checkpoint inhibition in the treatment of ovarian cancer. Gynecol Oncol Res Pract 2016;3:11. [Crossref] [PubMed]
- Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol 2016;34:3119-25. [Crossref] [PubMed]
- Berman D, Korman A, Peck R, et al. The development of immunomodulatory monoclonal antibodies as a new therapeutic modality for cancer: the Bristol-Myers Squibb experience. Pharmacol Ther 2015;148:132-53. [Crossref] [PubMed]
- Galsky MD, Hahn NM, Albany C, et al. Phase II trial of gemcitabine+ cisplatin+ ipilimumab in patients with metastatic urothelial cancer. J Clin Oncol 2016;34:abstr 357.
- Niu G, Chen X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr Drug Targets 2010;11:1000-17. [Crossref] [PubMed]
- Kim C, Yang H, Fukushima Y, et al. Vascular RhoJ is an effective and selective target for tumor angiogenesis and vascular disruption. Cancer cell 2014;25:102-17. [Crossref] [PubMed]
- Hahn NM, Stadler WM, Zon RT, et al. Phase II trial of cisplatin, gemcitabine, and bevacizumab as first-line therapy for metastatic urothelial carcinoma: Hoosier Oncology Group GU 04-75. J Clin Oncol 2011;29:1525-30. [Crossref] [PubMed]
- Balar AV, Apolo AB, Ostrovnaya I, et al. Phase II study of gemcitabine, carboplatin, and bevacizumab in patients with advanced unresectable or metastatic urothelial cancer. J Clin Oncol 2013;31:724-30. [Crossref] [PubMed]
- Falcon BL, Chintharlapalli S, Uhlik MT, et al. Antagonist antibodies to vascular endothelial growth factor receptor 2 (VEGFR-2) as anti-angiogenic agents. Pharmacol Ther 2016;164:204-25. [Crossref] [PubMed]
- Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [Crossref] [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [Crossref] [PubMed]
- Petrylak DP, Tagawa ST, Kohli M, et al. Docetaxel as monotherapy or combined with ramucirumab or icrucumab in second-line treatment for locally advanced or metastatic urothelial carcinoma: an open-label, three-arm, randomized controlled phase II trial. J Clin Oncol 2016;34:1500-9. [Crossref] [PubMed]
- Hughes PE, Caenepeel S, Wu LC. Targeted therapy and checkpoint immunotherapy combinations for the treatment of cancer. Trends Immunol 2016;37:462-76. [Crossref] [PubMed]
- Gabrilovich D, Ishida T, Oyama T, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 1998;92:4150-66. [PubMed]
- Lanitis E, Irving M, Coukos G. Targeting the tumor vasculature to enhance T cell activity. Curr Opin Immunol 2015;33:55-63. [Crossref] [PubMed]
- Wallin JJ, Bendell JC, Funke R, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun 2016;7:12624. [Crossref] [PubMed]
- McDermott DF, Sosman JA, Sznol M, et al. Atezolizumab, an anti–programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol 2016;34:833-42. [Crossref] [PubMed]
- Sonpavde G, Bellmunt J. Bladder cancer: Angiogenesis as a therapeutic target in urothelial carcinoma. Nat Rev Urol 2016;13:306-7. [Crossref] [PubMed]
- Sonpavde G, Jones BS, Bellmunt J, et al. Future directions and targeted therapies in bladder cancer. Hematol Oncol Clin North Am 2015;29:361-76. [Crossref] [PubMed]
- Tabernero J, Bahleda R, Dienstmann R, et al. Phase I dose-escalation study of JNJ-42756493, an oral pan–fibroblast growth factor receptor inhibitor, in patients with advanced solid tumors. J Clin Oncol 2015;33:3401-8. [Crossref] [PubMed]
- Rodriguez-Vida A, Saggese M, Hughes S, et al. Complexity of FGFR signalling in metastatic urothelial cancer. J Hematol Oncol 2015;8:119. [Crossref] [PubMed]
- Nogova L, Sequist LV, Perez Garcia JM, et al. Evaluation of BGJ398, a fibroblast growth factor receptor 1-3 kinase inhibitor, in patients with advanced solid tumors harboring genetic alterations in fibroblast growth factor receptors: Results of a global phase I, dose-escalation and dose-expansion study. J Clin Oncol 2017;35:157-65. [Crossref] [PubMed]
- Goyal L, Saha SK, Liu LY, et al. Polyclonal Secondary FGFR2 Mutations Drive Acquired Resistance to FGFR Inhibition in Patients with FGFR2 Fusion-Positive Cholangiocarcinoma. Cancer Discov 2017;7:252-63. [Crossref] [PubMed]
- Philips GK, Halabi S, Sanford BL, et al. A phase II trial of cisplatin (C), gemcitabine (G) and gefitinib for advanced urothelial tract carcinoma: results of Cancer and Leukemia Group B (CALGB) 90102. Ann Oncol 2009;20:1074-9. [Crossref] [PubMed]
- Hussain M, Daignault S, Agarwal N, et al. A randomized phase 2 trial of gemcitabine/cisplatin with or without cetuximab in patients with advanced urothelial carcinoma. Cancer 2014;120:2684-93. [Crossref] [PubMed]
- Hussain MH, MacVicar GR, Petrylak DP, et al. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu–positive urothelial carcinoma: Results of a multicenter phase II National Cancer Institute trial. J Clin Oncol 2007;25:2218-24. [Crossref] [PubMed]
- Cerbone L, Sternberg CN, Sengeløv L, et al. Results from a Phase I Study of Lapatinib with Gemcitabine and Cisplatin in Advanced or Metastatic Bladder Cancer: EORTC Trial 30061. Oncology 2016;90:21-8. [Crossref] [PubMed]
- Powles T, Huddart RA, Elliott T, et al. A phase II/III, double-blind, randomized trial comparing maintenance lapatinib versus placebo after first line chemotherapy in HER1/2 positive metastatic bladder cancer patients. J Clin Oncol 2015;33: abstr 4505.


