
Immune checkpoint inhibitors for metastatic bladder cancer
Introduction
Urothelial cancer of the bladder, renal pelvis, ureter and other urinary organs is the fourth most common cancer in males with more than 80,000 new cases expected and over 17,000 estimated deaths in the United States. The 5-year survival of patients with urothelial carcinoma (UC) varies according to the extension of the tumor, ranging from 70% in localized stages to 35% in tumours with close organs and/or lymph nodes involvement and decreases dramatically to 5% in metastatic stage (1).
Radical surgery remains the only curative approach for localized or locally advanced UC. Neo-adjuvant platinum-based chemotherapy can be offered in patients with locally advanced tumours and good performance status achieving a 5-year survival benefit of 5–7% (2,3). The role of adjuvant chemotherapy is still debated but several studies seem to show a disease-free survival (DFS) and overall survival (OS) benefit especially for high-risk patients treated with platinum regimens (4-6).
In patients with advanced unresectable or metastatic disease and good performance status, cisplatin combination regimens [cisplatin plus gemcitabine (CG), methothrexate, vinblastine, adriamycin and cisplatin (MVAC)] have represented the standard first line therapy whereas carboplatin-containing regimens or platinum-free combinations including taxanes and gemcitabine are generally reserved for patients unfit to receive cisplatin (7-14).
Despite these treatment options, the median overall survival (mOS) reached with cisplatin-based chemotherapy is only of 14 months. Furthermore, after progression to first line chemotherapy several drugs have been tested without significant improvement in mOS except for vinflunine which showed an OS benefit when compared to best supportive care (BSC) only in a subset of patients (15-17).
Chemotherapy has represented the standard therapy for locally advanced and metastatic UC for more than 30 years; however, the development of new immune-checkpoints inhibitors is dramatically changing the current treatment paradigm. Atezolizumab, a programmed death ligand 1 (PD-L1) inhibitor, was the first immune-agent approved in 2016 by FDA in patients with UC progressing on platinum-based chemotherapy.
To date, several other trials are currently exploring the role of these immune-agents in UC and the results are eagerly awaited
Immunotherapy in bladder cancer
The evidence that growth and progression of urothelial bladder cancer can be blocked by immune-system activation is well known. Indeed, early stage of urothelial bladder cancer with high risk of recurrence can be treated with instillation of Bacillus Calmette-Guérin (BCG) which through the recruitment of immune cells and the enhancement of inflammation response against tumour cells reduces the recurrence rate of the disease (18-21). More recently, Lawrence et al. demonstrated that bladder cancer, like melanoma and non-small cell lung cancer, is a tumour with a high somatic mutation frequency and with a high antigenic expression (22). As a result, bladder cancer is an optimal target to immune-checkpoint inhibitors which through an activation of immune-system could re-activate a suppressed immune response against urothelial cancer cells.
In this review we discuss the results of clinical trials exploring the efficacy of programmed death receptor and ligand 1 inhibitors as well as cytotoxic-T lymphocyte antigen 4 (CTLA 4) inhibitors focusing our attention on the new combination strategies and ongoing studies. Results of completed or partially completed clinical trials are described in Table 1 while a description of the main ongoing studies exploring PD-1/PD-L1 and CTLA-4 inhibitors are summarized in Table 2.
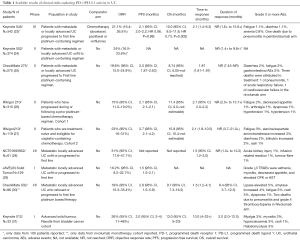
Full table
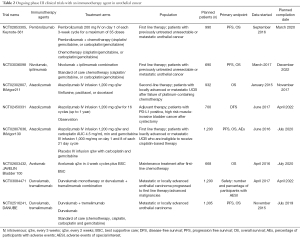
Full table
PD-L1 and PD-1
PD-L1 and programmed death receptor 1 (PD-1) are a member of the I Ig superfamily expressed on hematopoietic and non-hematopoietic cells (PD-L1) as well as on T Cell surface (PD-1). PD-L1/PD-1 interaction leads to “T-cell exhaustion” resulting in an impaired cytotoxic activity and decreased effector cytokine production, thus inhibiting the immune-response. Tumour cells can escape from immune pressure expressing PD-L1 on their surface and inducing PD-1 expression on T-cells (32-34).
Inhibition of both PD-L1 and PD-1 can restore and enhance immune activity against tumour cells.
Several PD-1 inhibitors (pembrolizumab and nivolumab) and PD-L1 inhibitors (atezolizumab, durvalumab and avelumab) have shown clinical efficacy in metastatic UC. In the next paragraphs we will describe the current evidences available from completed and ongoing clinical trials of each agent.
PD-1 inhibitors
Pembrolizumab
Pembrolizumab (MK-3475) is a humanized monoclonal IgG4 antibody against PD-1, which has already shown clinical activity in advanced melanoma, non-small cell lung cancer and head and neck squamous cell carcinoma. The first study assessing the role of pembrolizumab in urothelial tumours was the KEYNOTE-012, a phase Ib basket trial planned to investigate safety, tolerability and anti-tumour activity in patients with advanced gastric cancer, triple negative breast cancer, UC, and head and neck cancer. In the UC cohort, which included patients with locally advanced or metastatic urothelial cancer with transitional or non-transitional histology, pembrolizumab showed an interesting anti-tumour activity with acceptable safety profile (31).
Patients were required to have at least 1% of PD-L1 expression as detected on tumour or on tumour stroma by immunohistochemistry (IHC) performed by central laboratory. Key exclusion criteria included active autoimmune and interstitial lung disease as well as central nervous system (CNS) metastases. Among 115 patients screened, 61 were PD-L1 positive and 33 patients were enrolled in the study and received at least one cycle of pembrolizumab at a dosage of 10 mg/kg every 2 weeks. Patients evaluable for response were 27 out of 33.
The most common treatment-related adverse events were fatigue (18%) and peripheral oedema (12%). Of note, 5 patients experienced a grade 3 toxicity, which resulted in treatment discontinuation for 2 patients (one patient developed myositis and rhabdomyolysis while the other grade 3 hypercalcaemia). Regarding clinical activity, after a median follow up of 10 months, 3 out of 27 patients in the full analysis set achieved a complete response (CR: 11%), 4 a partial response (PR: 15%), 4 a stable disease (SD: 15%) and 14 had progressive disease (PD: 52%). The median time to response was 2 months (range, 2–13 months) while the median duration of response was 10 months (range, 4–22 months).
Of note, correlation with PD-L1 expression and response was observed when determination of PD-L1 were performed on tumour cells and tumour-associated inflammatory cells suggesting the creation of a combined score involving PD-L1 determination on tumour and tumour-related immune cells for future trial exploring pembrolizumab.
On the wave of the positive results obtained in KEYNOTE-012 pembrolizumab is currently being tested as first line therapy in patients unfit to cisplatin (24).
The KEYNOTE-052 is a phase II trial in which 374 patients with locally advanced or metastatic UC (with transitional histology) and cisplatin ineligibility have been enrolled to receive pembrolizumab 200 mg every 3 weeks (q3w). Primary study endpoint is the overall response rate (ORR) as assessed by an independent review centre in all population and in PD-L1 positive patients with PD-L1 expression assessed on tumour and immune related stroma [combined positive score (CPS)]. To date, results of a planned interim analysis performed on 100 patients have shown an interesting ORR (24% of overall responses with 6% of CR) suggesting also a correlation between PD-L1 expression and response rate (patients with CPS ≥10% had an ORR of 36.7% with 13.3% of CR). Future analysis on the overall treated population will give more information about the role of pembrolizumab in first line therapy in patients unfit to platinum-based therapy.
The role of pembrolizumab has also been investigated in second-line setting and the results of a large randomized phase III clinical trial (KEYNOTE-045) have showed a significant clinical activity of pembrolizumab compared with chemotherapy (23). In this trial, 542 patients with locally advanced or metastatic predominantly transitional UC and progressing on first line platinum-based regimen or with disease recurrence within 12 months after adjuvant or neoadjuvant platinum containing therapy, were randomized (1:1) to receive pembrolizumab 200 mg q3w or chemotherapy (paclitaxel 175 mg/m2 q3w or docetaxel 75 mg q3w or vinflunine 320 mg/m2 q3w). Exclusion criteria were active CNS metastases and/or carcinomatous meningitis, prior therapy with PD-1/PD-L1 inhibitor and diagnosis of immunodeficiency. Patients with Eastern Cooperative Oncology Group (ECOG) performance status 2 without poor prognostic factors (haemoglobin <10 g/dL, presence of liver metastases and most recent chemotherapy within 3 months before enrolment) were allowed. In this study progression free survival (PFS) and OS in overall population and in patients with PD-L1 CPS ≥10% were co-primary endpoints while ORR, safety and duration of confirmed response were secondary outcomes. Results of this study showed a better OS for patients treated with pembrolizumab in the overall population (mOS 10.3 vs. 7.4 months; HR 0.73; 95% CI, 0.59–0.91, P=0.002) and in patients with CPS ≥10% (mOS 8.0 vs. 5.2 months; HR 0.57; 95% CI, 0.37–0.88, P=0.0048). No differences in terms of PFS were found between the two arms. Overall response rate as assessed on the intention-t-treat population (n=270 and 273 in pembrolizumab and chemotherapy arms respectively) was significantly higher in the pembrolizumab group both in overall population (21.1% vs. 11.4%) and in CPS ≥10% population (21.6% vs. 6.7%) with a median time to response of 2.1 months (range, 1.4–6.3) and duration of response not reached (1.6–15.6+ months). Pembrolizumab also showed a good safety profile with the most common adverse events being fatigue (13.5%), pruritus (19.5%), nausea (10.9%) and diarrhoea (9.0%). Of note, one treatment-related death due to pneumonitis was observed in pembrolizumab arm. As a prolonged duration of response was seen only in patients who had response to pembrolizumab and because these responses occurred in less than half of the intention-to-treat population, no advantages in terms of median PFS were observed in the pembrolizumab arm. Furthermore, benefit of pembrolizumab appeared to be independent of PD-L1 expression as assessed on CPS.
Pembrolizumab has therefore demonstrated to improve both OS and ORR with a better safety profile compared with chemotherapy in previously platinum-treated patients with UC, and it’s very likely that it will be a second line option in the next future.
Based on these results, pembrolizumab is currently being investigated also in other settings of the disease. Indeed, a phase II trial (NCT02500121) is currently testing pembrolizumab as maintenance therapy after standard first line chemotherapy in patients with locally advanced or metastatic UC. Primary endpoint of the study is 6-month PFS. A total of 200 patients who have not progressed on first-line platinum-based chemotherapy, will be randomized to receive pembrolizumab or placebo. Another phase II trial (NCT02736266) will explore the role of this PD-1 inhibitor as neo-adjuvant treatment in 90 patients with muscle invasive urothelial bladder carcinoma (T2–T4 N0) with residual disease after transurethral resection. Patients enrolled will receive 3 cycles of pembrolizumab (200 mg q3w) before planned cystectomy. Primary endpoint is pathological complete response rate. Regarding the role of immune-therapy in early-stage disease, a phase II trial (NCT02625961) will test pembrolizumab in 260 patients with high-risk non-muscle invasive bladder cancer (high risk Ta, T1, carcinoma in situ) refractory to BCG therapy with primary endpoints being DFS and pathological complete response rate.
Nivolumab
Nivolumab (MDX 1106) is a fully human IgG4 monoclonal antibody against PD-1 which has been approved for treatment of advanced melanoma, Hodgkin lymphoma, non-small cell lung cancer, head and neck cancer, and renal cell cancer.
The multicentre phase 1/2, open-label, two-stage, multi-arm study CheckMate 032 was the first trial assessing the activity of nivolumab in patients with UC (30). This trial patients were could receive nivolumab alone (3 mg/kg every 2 weeks) or in combination with ipilimumab. To date, only the results of the nivolumab monotherapy arm (3 mg/kg every 2 weeks) are available. In this cohort, patients with metastatic or locally advanced UC who had progressed to at least one previous platinum-based chemotherapy were enrolled. Of note, patients were not selected based on tumour PD-L1 expression. Key exclusion criteria included active CNS metastases, history or active autoimmune disease, treatment with immunosuppressive dose of corticosteroids (>10 mg per day) and previous therapies with immuno-agents. After progression to nivolumab monotherapy patients could switch to combination arms (nivolumab 1 mg/kg + ipilimumab 3 mg/kg or nivolumab 3 mg/kg + ipilimumab 1 mg/kg). Primary endpoint of the study was ORR. Among 86 patients screened, 78 were assigned to nivolumab and received at least one dose of study drug. After a median follow-up of 15.2 months, 30 patients had a PD (38%), 5 a CR (6%), 14 a PR (18%) and 22 (28%) achieved a SD as best response (7 patients were non evaluable). Median time to response was 1.5 months (range, 1.2–4.1 months) and median duration of response was 9.4 months (range, 5.7–12.5 months). Grade 3 or 4 treatment related adverse events were: lipase elevation (5%), amylase elevation (4%), fatigue, maculopapular rash, dyspnoea, decreased lymphocyte and neutrophil count. Of note, 2 patients died due to treatment related adverse events (grade 4 pneumonitis and grade 4 thrombocytopenia). No correlation between PD-L1 expression, which was assessed retrospectively, and response rate has been observed in this study. Despite the short follow-up and the small sample size of this study, nivolumab showed for the first time to be an active treatment for patients with recurrent UC justifying the planning of further larger trials exploring nivolumab in this setting.
CheckMate 275 is a multicentre, single-arm, phase 2 trial where nivolumab has been tested in 270 patients with metastatic or unresectable locally advanced UC progressed after at least one platinum-based regimen or with recurrent disease within 12 months of neo-adjuvant or adjuvant platinum-based chemotherapy (25). Patients who received more than 2 lines of therapy were excluded. All patients enrolled received at least one dose of nivolumab 3 mg/kg q2w. PD-L1 expression on tumour cells was determined at screening but was not an eligibility criterion. Primary endpoint was ORR in all treated population and in patients with PD-L1 expression of 5% or greater or 1% or greater. Among the 265 patients included in the activity analysis, 52 achieved a confirmed objective response resulting in an ORR of 19.6% with 6 achieving a CR (2%), 46 a PR (17%), 60 a SD (23%). Of note, analysis of confirmed objective response was not performed on 49 patients (18%) mainly due to patient death before the first scan. Among patients with PD-L1 expression of 5% or greater (n=81), 23 had a confirmed response (28.4%) while 29 patients (23.8%) of the 122 patients with PD-L1 expression ≥1% achieved an objective response. Time to response was 1.87 months (range, 1.81–1.97) while duration of response was not reached (range, 7.43–NR). Grade 3 or 4 treatment related AEs occurred in 48 patients (18%) with diarrhoea (2%), fatigue (2%), rash (1%) and asthenia (1%). Three deaths were attributed to treatment (one patient died of pneumonitis, one of acute respiratory failure and one of cardiovascular failure). Median PFS was 2.0 months (95% CI, 1.87–2.63) while median OS was 8.74 months (95% CI, 6.05–not reached) in overall population, 11.30 months (8.74–not reached) in patients with PD-L1 expression of 1% or greater and 5.95 months (4.30–8.08 months) in patients with PD-L1 expression less than 1%.
Results of this study demonstrated a significant benefit in ORR with nivolumab for previously treated patients with metastatic or locally advanced UC. Indeed, the ORR reached in this trial was 19.6% in overall population which was significantly higher than the planned comparator of 10% (based on historical results with chemotherapy).
Regarding the population with PD-L1 expression of 5% or 1% or greater, Authors planned a target objective response rate of 30% which has not been reached in this study (28.4% in PD-L1 ≥5% and 23.8% in PD-L1 ≥1% population) suggesting that PD-L1 expression may not be a predictive biomarker to nivolumab. Interestingly, based on the hypothesis that interferon-γ signalling is associated to resistance to nivolumab in melanoma patients (35,36), authors have compared nivolumab response to gene expression in tumour tissue: the results showed that interferon-γ gene expression and UC molecular subtype are associated with nivolumab response. Despite OS being a secondary endpoint of this study, results suggest a survival benefit with nivolumab which could be more considerable in PD-L1 expressing patients. This hypothesis should be further investigated in larger and randomized clinical trials aiming to assess whether nivolumab is actually associated with a survival advantage in patients with previously treated metastatic or locally advanced UC.
Based on these positive results, several trials exploring nivolumab alone or in combination with different agents (see below) in patients with UC are currently ongoing in different disease settings.
Of note, single agent nivolumab is being tested in a large randomized placebo-controlled phase III clinical trial evaluating its role as adjuvant treatment in patients with high risk invasive urothelial cancer (NCT02632409). The planned enrolment is 640 patients with primary endpoint being DFS. The study started in February 2016 with estimated primary completion date in October 2020.
PD-L1 inhibitors
Atezolizumab
Atezolizumab (MPDL3280A) is an engineered, humanised monoclonal IgG1 antibody, with a high affinity for PD-L1 acting as inhibitor of the interaction between PD-L1 and PD1/B7.1.
In a phase I study (NCT01375842), 67 patients with metastatic urothelial cancer were enrolled to receive atezolizumab at the dose of 15 mg/kg IV every three weeks for 16 cycles and for a total treatment time of one year. Patients were scored according to IHC status with a range between 0 and 3. An objective response was seeing in 43% patients with IHC score 2/3 tumours and in 11% patients with IHC score 0/1 tumours (37).
A following phase II trial (IMvigor 210 trial, NCT02108652) with two different cohorts was performed: cohort 1 included patients with metastatic urothelial cancers ineligible for first-line platinum-based chemotherapy whereas cohort 2 enrolled patients who progressed during or following platinum-based treatment. Three hundred and ten [310] patients were enrolled in cohort 2 and received a fixed dose of 1200 mg intravenous (IV) atezolizumab; 74% of the patients had urinary bladder as primary tumour site, while 14% and 7% of the patients had renal pelvis and ureter as primary tumour site, respectively. The co-primary endpoints were the independent review facility-assessed objective response rate according to RECIST version 1.1 and the investigator-assessed objective response rate according to immune-modified RECIST (analysed by intention-to-treat).
The analysis showed an objective response rate of 15% and a 12-month OS of 37% in the overall population. The most common AEs in the overall population were fatigue (31%) and nausea (14%). The rate of grade 3 and 4 adverse events was 16%, with a very low rate of discontinuation from the study due to adverse events.
Tumour samples were assessed prospectively and centrally for PD-L1 expression by IHC with the SP142 assay (Ventana, AZ, USA). PD-L1 expression ≤1% in tumour-infiltrating immune cells (IC) was defined as IC0; tumours were defined IC1 if PD-L1 was expressed on ≥1% and <5% of the IC and IC2/3 if PD-L1 was expressed on ≥5% of the IC. The results showed a 27% of ORR and a 50% of 12-month OS in patients with IC2/3 tumours. Complete response occurred in 5% of the all patients and in 11%, 2% and 2% of patients with IC2/3, IC1 and IC0 tumours, respectively. The responses were generally durable, with the median duration of response not reached after a medium of 17.5 months of follow-up (26).
Based on the favourable results of cohort 2, atezolizumab has been approved by FDA under accelerated approval for patients with locally advanced or metastatic UC who have progressed during or following platinum-based chemotherapy or whose disease has worsened within 12 months after neoadjuvant or adjuvant platinum-based chemotherapy (38). It is the first new drug approved for metastatic UC in over 30 year and it is also pending approval in Europe.
In cohort 1 of IMvigor 210 trial, 119 chemotherapy-naïve metastatic patients who were unfit to receive cisplatin were enrolled. Main criteria used to define a patient ineligible for cisplatin-based chemotherapy were: impaired renal function (glomerular filtration rate 30–60 mL/min), a hearing loss of 25 dB at two contiguous frequencies, grade 2 or more peripheral neuropathy, or ECOG performance score of ≥2. The treatment regimen was the same as in cohort 2 and the patients were grouped by PDL1-expression status on ICs (33% of the patients were IC0, 40% were IC1, and 27% were IC2/3).
The ORR, primary endpoint of this study, for the whole cohort was 23%, with 7% CR and 17% PR, and ORR was 21% in IC0 group, 24% in IC1 group, and 28% in IC2/3 group. Responses were durable, with the median duration of response not reached at 14.4 months of follow-up. Median OS was 14.8 months (95% CI, 10.1 months to not reached) and 57% of the patients were alive at 12 months. Overall survival in the IC0/1 groups was similar to OS achieved in the IC2/3 groups (15.3 vs. 12.3 months, respectively) (27).
On the wave of these positive results, several other trials are testing atezolizumab in different stages of UC.
A large phase III trial (NCT02302807) will randomize 932 patients with metastatic or unresectable UC progressed to standard platinum-based chemotherapy to receive atezolizumab monotherapy or second line chemotherapy (vinflunine, paclitaxel or docetaxel). Primary endpoint of this study is OS with enrolment planned to end by November 2017.
The role of this PD-L1 inhibitor in adjuvant setting is under investigation in a large phase III (NCT02450331) exploring the different DFS in 700 patients with histologically confirmed muscle invasive UC of the bladder or upper urinary tract. Patients will be randomized to receive atezolizumab (1,200 mg q3w for 16 cycles) or observation. The planned completion date is in April 2022. Furthermore, other phase II trials are currently exploring the role of atezolizumab in patients with high grade urothelial bladder cancer refractory to BCG (NCT02844816) as well as preoperative treatment in patients with T2–T4a urothelial bladder cancer (NCT02662309) and in those refusing or unfit for platinum based therapy (NCT02451423).
Avelumab
Avelumab (MSB0010718C) is a fully human IgG1 anti-PD-L1 monoclonal antibody with potential antibody-dependent cell-mediated cytotoxicity properties which has been tested in more than 15 different types of cancers, including bladder (39). A phase I trial (NCT01772004) with avelumab (10 mg/kg IV q2w) was carried out in 129 patients, including 9 who were platinum-ineligible and 113 who progressed after platinum-based therapy. Recent results from the ongoing JAVELIN Solid Tumor phase 1b trial were presented at the 2016 European Society for Medical Oncology (ESMO) annual meeting and showed that, after at least 4 months of follow-up in 109 patients, the ORR was 16.5%, with 3 CRs and 15 PRs. The median PFS was 6.1 weeks and the PFS rate at 12 weeks was 35.6%. After a median of 10.4 weeks of treatment, 60.5% of patients had a treatment-related adverse event, including infusion-related reaction and fatigue. Only one death occurred, due to pneumonitis (40). An early analysis of patients with PD-L1 positive tumours (PD-L1 expression assessed by IHC ≥5%), presented at 2016 Genitourinary Cancers Symposium, showed a trend towards higher ORR and prolonged PFS rate at 12 weeks in patients with PD-L1 positive metastatic UC (29).
The phase III trial JAVELIN Bladder 100 study (NCT02603432) is currently ongoing to comparing maintenance treatment with avelumab plus BSC versus BSC alone in metastatic UC patients who have not progressed during or following first-line systemic therapy. Primary endpoint is OS and approximately 668 patients are planned to be enrolled. This study is expected to be completed in July 2019.
Durvalumab
Durvalumab is a selective, high-affinity, human immunoglobulin G1 k monoclonal antibody against PD-L1. It is in the early stage of development for the treatment of non-small cell lung cancer, head and neck cancer, gastric cancer, pancreatic cancer, hepatocellular carcinoma, mesothelioma, hematologic cancers and urothelial cancers.
A phase I/II trial (NCT01693562) is being conducted in 61 patients with inoperable or metastatic urothelial bladder cancer who are ineligible or progressed on first-line therapy to evaluate the safety and anti-tumour activity of durvalumab monotherapy (10 mg/kg every 2 weeks for up to 12 months) (28). Patients were heavily pretreated, with 93.4% patients who had received one or more prior lines of chemotherapy for metastatic disease and 31.1% who had received three or more prior therapies. In the overall population, the ORR was 31% (95% CI, 17.6 to 47.1) and the median duration of response has not yet been reached (4.1 to 49.3 weeks). Durvalumab was evaluated also according to PD-L1 expression on tumour cells (TC) and IC. PD-L1 was defined as positive if either ≥25% of TC or ≥25% of IC expressed PD-L1 whereas PD-L1 was defined as negative if both <25% of TC and <25% of IC expressed PD-L1. The ORR was 46% in the PD-L1 positive subgroup and 0% in the PD-L1 negative subgroup. The disease control rate at 12 weeks was 57.1% and 28.6% respectively. The most common adverse events were fatigue (13%), diarrhoea (10%) and decrease appetite (8%); grade 3 AEs occurred in three patients (acute kidney injury, infusion-related reaction, tumour flare), and grade 4 or 5 adverse events were not reported. This trial is currently ongoing and results of a larger cohort of patients are expected in July 2017.
An ongoing phase 1 open-label (NCT02118436) study is recruiting patients with relapsed/refractory advanced solid malignancies to receive durvalumab in combination to MEDI0680, a humanized IgG4κ monoclonal antibody specific for human PD-1 that blocks interaction with PD-L1 and programmed cell death ligand-2 (PD-L2). Preliminary results showed a 15% ORR and a 35% DCR. The most common drug-related adverse events were pruritus (17%), fatigue (13%), diarrhoea (13%), flushing (10%), peripheral edema (10%) and pyrexia (10%) (41).
CTLA-4 inhibitors
The CTLA-4 also known as CD156 is a member of the immunoglobulin superfamily and a transmembrane receptor expressed exclusively on T cells. CTLA-4 activation depends on the interaction with CD80 and CD86 on antigen presenting cells (APC) and leads to a down-regulation of T-helper lymphocyte and enhanced regulatory T cell immunosuppressive activity resulting in immune response inhibition.
The counterpart of CTLA-4 is the CD28 protein which, once activated, strongly amplifies T-Cell activation and amplification. Although CTLA-4 is homologous to CD28, it binds to CD80 and CD86 with more affinity (42).
The evidence that CTLA-4 inhibition results in enhanced immune-response against tumours has led to the development of specific CTLA-4 inhibitors who have showed significant results in melanoma (43-45).
Few clinical trials have evaluated the role of CTLA-4 inhibitors in UC, but it’s very likely that several ongoing studies with CTLA-4 inhibitors (ipilimumab and tremelimumab) alone or in combination strategies will provide further information about their role.
Ipilimumab
Ipilimumab (MDX-010) is a fully human IgG1 monoclonal antibody directed against CTLA-4. To date, the only available data on its role in UC come from a small study done by Carthon et al. (46). In this trial 12 patients with localized (T1–2, N0, M0) UC received two cycles of different dosages of ipilimumab (3 mg/kg q3w, n=6 or 10 mg/kg q3w n=6) before planned cystectomy. The study was designed to understand the safety profile of ipilimumab in this subgroup of patients as well as to define the immunologic profile in peripheral blood related with tumour response to treatment. Of the 12 patients treated with ipilimumab before surgery, 8 showed a lower stage on pathological evaluation. This evaluation could have been influenced by the transurethral resection done before ipilimumab treatment. Nonetheless, urine cytological and FISH assessment showed that four patients had change from positive urine cytology/FISH to negative cytology/FISH urine analysis after ipilimumab treatment. Grade ≥3 adverse events related to ipilimumab mainly consisted of diarrhoea (2 patients in ipilimumab 10mg/kg arm) and ischemic papillopathy with optic neuritis (1 patient in ipilimumab 3 mg/kg arm). Authors concluded that ipilimumab showed a tolerable safety profile in preoperative setting, suggesting that the development of further trials in this setting would provide further information about ipilimumab activity and biological data regarding human immune response after immune-checkpoint inhibition.
Future development of immune-checkpoint inhibitors
The approval of immune-checkpoints inhibitors in patients with UC has led to revolution that will profoundly change the treatment paradigm of this disease.
Despite these encouraging results, it appears clear that not all the patients with UC respond to immunotherapy. However, several strategies aimed to enhance the therapeutic potential of immune-checkpoint inhibitors are under investigation. Indeed, tumours adopt several mechanisms through which become ‘invisible’ to immune cells. The absence of priming signals, the activation of tolerance signals by suppressive cytokines or recruitment of regulatory T cells, the absence of antigens or the absence of APCs and the stromal interactions are possible strategies adopted by cancer cells to escape from immune-pressure (47-49).
Several strategies such as combination therapy between PD-1/PD-L1 and CTLA-4 inhibitors or immune-checkpoint inhibitors and target agents, chemotherapy and/or radiotherapy are being tested to overcome the tumour-immune-escape in different setting of the disease.
As already discussed before, PD-L1/PD-1 and CTLA-4 inhibitors adopt a different mechanism to enhance immune-response against tumours working in different pathways of a common cascade resulting in T cell activation.
This combination strategy has been already tested in melanoma with significant improvement of long-term responses compared to single agent monotherapy (50,51).
The combination of nivolumab and ipilimumab is currently being tested in a large randomized clinical trial where patients with untreated metastatic or unresectable UC will be randomized to receive the immune checkpoints combination or platinum-based chemotherapy (NCT03036098; CheckMate 901). The estimated enrolment of this trial is 690 patients with PFS and OS as primary endpoint.
An ongoing phase III trial (NCT02516241; DANUBE) of durvalumab as a monotherapy or combined with tremelimumab (CTLA4 inhibitor) versus standard-of-care chemotherapy (gemcitabine plus cisplatin or gemcitabine plus carboplatin) is currently recruiting patients with metastatic or unresectable UC. This 3-arm trial is expected to be completed in 2019 and OS is the primary endpoint.
Another phase III clinical trial (NCT03084471; STRONG) is currently comparing the combination durvalumab-tremelimumab with durvalumab monotherapy in advanced solid malignancies with an estimated enrolment of 1,200 patients. The aim of this study is to evaluate the safety profile of the combination immune-therapy with an estimated completion data in April 2022. Of note, the combination of durvalumab with tremelimumab is under investigation also in patients with muscle-invasive, high-risk UC who are ineligible for cisplatin-based neoadjuvant chemotherapy (NCT02812420).
Radiotherapy and chemotherapy could play a key role to overcome immune-tumour escape. Indeed, tumour irradiation stimulates an intensive inflammation in the site of application, promotes the production of adhesion molecules and MHC I and activates an intensive flux of CD8+ lymphocytes (52-56). Through these mechanisms, irradiation drives an immune response that leads also to the regression of distant and un-irradiated tumour lesions (abscopal effect) (57,58). As such, it should come as no surprise that combination of immune-checkpoint inhibitors and radiotherapy represents an emerging strategy with a strong biological rationale which is currently being tested in several phase I/II clinical trials (58,59).
Chemotherapy has represented the standard therapy for metastatic or locally advanced unresectable UC and may play an important role also in association with immune-checkpoints inhibitors. There are several proposed mechanisms through which chemotherapy could overcome immune-tumour escape. First the lytic effect done by antiblastic agents could lead to the presentation of antigen and neo-antigen resulting in T-cell activation. Second, the elimination of immunosuppressive cells could restore immune activity against tumours. Third, chemotherapy could drive a better penetration of immune cells in tumour stroma. Furthermore, there are data suggesting that patients who received chemotherapy after immune treatment could have a better response to antiblastic agents indicating a synergic effect between chemotherapy and immune-therapy (60-63). This approach is currently being investigated by several clinical trials in UC. Of note, two phase III clinical trials are exploring the association between pembrolizumab or atezolizumab and chemotherapy. The KEYNOTE-361 (NCT02853305) is a phase III randomized clinical trial with an estimated enrolment of 990 patients with metastatic or locally advanced UC who will be randomized to receive pembrolizumab (200 mg q3w) with or without platinum-based chemotherapy or chemotherapy alone. PFS and OS are primary endpoints with estimated primary data expected in March 2019 and the study is expected to end in April 2020.
The IMvigor130 (NCT02807636) is a randomized placebo-controlled phase 3 clinical trial where 1,200 patients with metastatic or locally advanced unresectable urothelial cancer will be randomized to receive atezolizumab (1,200 mg q3w) alone or in combination with platinum-based chemotherapy. Patients enrolled in the control arm will receive standard platinum-based chemotherapy with placebo. This study will test safety and the clinical efficacy of atezolizumab monotherapy or in combination with primary endpoints being PFS, OS and adverse events rate. The estimated primary completion data is expected in December 2018 while the completion of study in July 2020.
Conclusions
With chemotherapy being the standard of care for UC in the last years, immunotherapy will probably represent a new era. Results of clinical trials discussed in this review have demonstrated that immunotherapy is a realistic hope for patients in different settings of this disease. Indeed, as observed in other tumours, also in UC, immunotherapy could lead to durable and stable responses. Moreover, due to an overall acceptable safety profile, it will be a treatment that could be offered to patients ineligible to platinum therapy.
Ongoing studies will clarify which patients could benefit the most from these therapies, as well as if there are combination strategies able to increase the number of responders and to improve clinical outcomes.
Urothelial carcinoma treatment paradigm is rapidly evolving and immunotherapy revolution has just begun.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ja Hyeon Ku, Kunyoo Shin, Minyong Kang) for the series “Bladder Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.05.36). The series “Bladder Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Winquist E, Kirchner TS, Segal RGenitourinary Cancer Disease Site Group, et al. Cancer Care Ontario Program in Evidence-based Care Practice Guidelines Initiative. J Urol 2004;171:561-9. [Crossref] [PubMed]
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta- analysis collaboration. Eur Urol 2005;48:202-5. [Crossref] [PubMed]
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Adjuvant chemotherapy for invasive bladder cancer (individual patient data). Cochrane Database Syst Rev 2006;2:CD006018 [PubMed]
- Leow JJ, Martin-Doyle W, Rajagopal PS, et al. Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol 2014;66:42-54. [Crossref] [PubMed]
- Seisen T, Krasnow RE, Bellmunt J, et al. Effectiveness of Adjuvant Chemotherapy After Radical Nephroureterectomy for Locally Advanced and/or Positive Regional Lymph Node Upper Tract Urothelial Carcinoma. J Clin Oncol 2017;35:852-60. [Crossref] [PubMed]
- Logothetis CJ, Dexeus FH, Finn L, et al. A prospective randomized trial comparing CISCA to MVAC chemotherapy in advanced metastastic urothelial tumors. J Clin Oncol 1990;8:1050-5. [Crossref] [PubMed]
- von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000;18:3068-77. [Crossref] [PubMed]
- von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005;23:4602-8. [Crossref] [PubMed]
- Sternberg CN, de Mulder PH, Schornagel JH, et al. Randomized Phase III Trial of high dose intensity Methotrexate, Vinblastine, Doxorubicin, and Cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer. Protocol No. 30924. J Clin Oncol 2001;19:2638-46. [Crossref] [PubMed]
- Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumors (EORTC protocol 30924). Eur J Cancer 2006;42:50-54. [Crossref] [PubMed]
- De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 2012;30:191-9. [Crossref] [PubMed]
- Galsky MD, Chen GJ, Oh WK, et al. Comparative effectiveness of cisplatin-based and carboplatin-based chemotherapy for treatment of advanced urothelial carcinoma. Ann Oncol 2012;23:406-10. [Crossref] [PubMed]
- Locke JA, Pond GR, Sonpavde G, et al. Efficacy and Safety of Gemcitabine Plus Either Taxane or Carboplatin in the First-Line Setting of Metastatic Urothelial Carcinoma: A Systematic Review and Meta-Analysis. Clin Genitourin Cancer 2016;14:331-40. [Crossref] [PubMed]
- Raggi D, Miceli R, Sonpavde G, et al. Second-line single-agent versus doublet chemotherapy as salvage therapy for metastatic urothelial cancer: a systematic review and meta-analysis. Ann Oncol 2016;27:49-61. [Crossref] [PubMed]
- Bellmunt J, Théodore C, Demkov T, et al. Randomised phase III trial of vinflunine (V) plus best supportive care (B) compared with B alone a after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 2009;27:4454-61. [Crossref] [PubMed]
- Bellmunt J, Fougeray R, Rosenberg JE, et al. Long-term survival results of a randomized phase III trial of vinflunine plus best supportive care versus best supportive care alone in advanced urothelial carcinoma patients after failure of platinum- based chemotherapy. Ann Oncol 2013;24:1466-72. [Crossref] [PubMed]
- Sylvester RJ, van der Meijden AP, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol 2002;168:1964-70. [Crossref] [PubMed]
- Böhle A, Bock PR. Intravesical bacille Calmette-Guérin versus mitomycin C in superficial bladder cancer: formal meta- analysis of comparative studies on tumor progression. Urology 2004;63:682-6. [Crossref] [PubMed]
- Malmström PU, Sylvester RJ, Crawford DE, et al. An individual patient data meta-analysis of the long-term outcome of random- ised studies comparing intravesical mitomycin C versus bacillus Calmette-Guerin for non-muscle-invasive bladder cancer. Eur Urol 2009;56:247-56. [Crossref] [PubMed]
- Sylvester RJ, Brausi MA, Kirkels WJ, et al. Long term efficacy results of EORTC genito-Urinary Group randomized phase 3 study 30911 comparing intravescical instillation of epirubicin, bacillus Calmette-guerin, and bacillus Calmette- Guerin plus isoniazid in patients with intermediate and high risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol 2010;57:766-73. [Crossref] [PubMed]
- Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013;499:214-8. [Crossref] [PubMed]
- Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 2017;376:1015-26. [Crossref] [PubMed]
- Balar, J. Bellmunt, P.H. O'Donnell, et al. Pembrolizumab (pembro) as first-line therapy for advanced/unresectable or metastatic urothelial cancer: Preliminary results from the phase 2 KEYNOTE-052 study. Ann Oncol 2016;27:abstr LBA32_PR.
- Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312-22. [Crossref] [PubMed]
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909-20. [Crossref] [PubMed]
- Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin ineligible patients with locally advanced andmetastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67-76. [Crossref] [PubMed]
- Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol 2016;34:3119-25. [Crossref] [PubMed]
- Apolo AB, Infante JR, Hamid O, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with metastatic urothelial carcinoma from the JAVELIN solid tumor phase 1b trial: Analysis of safety, clinical activity, and PD-L1 expression. J Clin Oncol 2016;34:abstr 4514.
- Sharma P, Callahan MK, Bono P, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol 2016;17:1590-8. [Crossref] [PubMed]
- Plimack ER, Bellmunt J, Gupta S, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol 2017;18:212-20. [Crossref] [PubMed]
- Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother 2007;56:739-45. [Crossref] [PubMed]
- Schepisi G, Santoni M, Massari F, et al. Urothelial Cancer: Inflammatory Mediators and Implications for Immunotherapy. BioDrugs 2016;30:263-73. [Crossref] [PubMed]
- Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 2015;15:486-99. [Crossref] [PubMed]
- Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med 2016;375:819-29. [Crossref] [PubMed]
- Gao J, Shi LZ, Zhao H, et al. Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell 2016;167:397-404.e9. [Crossref] [PubMed]
- Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558-62. [Crossref] [PubMed]
- Inc. G. TECENTRIQ (atezolizumab) injection. Prescribing Information 2016. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761034s000lbl.pdf
- Boyerinas B, Jochems C, Fantini M, et al. Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res 2015;3:1148-57. [Crossref] [PubMed]
- Patel MR, Ellerton J, Agrawal M, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with metastatic urothelial carcinoma progressed after platinum-based therapy or platinum ineligible. Ann Oncol 2016;27:266-95. [Crossref]
- Hamid O, Chow LQ, Sanborn RE, et al. Combination of MEDI0680, an anti-PD-1 antibody, with durvalumab, an anti-PD-L1 antibody: a phase 1, open-label study in advanced malignancies. Ann Oncol 2016;27:abstr 1050PD.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Massari F, Ciccarese C, Vau N, et al. Emerging Immunotargets in Bladder Cancer. Curr Drug Targets 2016;17:757-70. [Crossref] [PubMed]
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N Engl J Med 2016;375:1845-55. [Crossref] [PubMed]
- Carthon BC, Wolchok JD, Yuan J, et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res 2010;16:2861-71. [Crossref] [PubMed]
- Chen PL, Roh W, Reuben A, et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov 2016;6:827-37. [Crossref] [PubMed]
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541:321-30. [Crossref] [PubMed]
- Ott PA, Hodi FS, Kaufman HL, et al. Combination immunotherapy: a road map. J Immunother Cancer 2017;5:16. [Crossref] [PubMed]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23-34. [Crossref] [PubMed]
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006-17. [Crossref] [PubMed]
- Matsumura S, Wang B, Kawashima N, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol 2008;181:3099-107. [Crossref] [PubMed]
- Lugade AA, Sorensen EW, Gerber SA, et al. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol 2008;180:3132-9. [Crossref] [PubMed]
- Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006;203:1259-71. [Crossref] [PubMed]
- Ruocco MG, Pilones KA, Kawashima N, et al. Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects. J Clin Invest 2012;122:3718-30. [Crossref] [PubMed]
- Chakraborty M, Abrams SI, Coleman CN, et al. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res 2004;64:4328-37. [Crossref] [PubMed]
- Hodge JW, Sharp HJ, Gameiro SR. Abscopal regression of antigen disparate tumors by antigen cascade after systemic tumor vaccination in combination with local tumor radiation. Cancer Biother Radiopharm 2012;27:12-22. [Crossref] [PubMed]
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [Crossref] [PubMed]
- Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373-7. [Crossref] [PubMed]
- Apetoh L, Vegran F, Ladoire S, et al. Restoration of antitumor immunity through selective inhibition of myeloid derived suppressor cells by anticancer therapies. Curr Mol Med 2011;11:365-72. [Crossref] [PubMed]
- Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol 2011;8:151-60. [Crossref] [PubMed]
- Schlom J, Arlen PM, Gulley JL. Cancer vaccines: moving beyond current paradigms. Clin Cancer Res 2007;13:3776-82. [Crossref] [PubMed]
- Wheeler CJ, Das A, Liu G, et al. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res 2004;10:5316-26. [Crossref] [PubMed]


