Continuous intraoperative neuromonitoring in minimally invasive video assisted thyroid surgery: first experience
Introduction
Intraoperative neuromonitoring (ioNM) is used in various fields. IoNM was developed to monitor the functional nerval integrity, with the aim to detect or prevent impending/imminent damage due to indirect or direct nerve injury at an early stage. Alteration of the neural response during surgery may lead to a change in operative strategy to prevent further and potentially irreversible damage. ioNM is widely used in thyroid and parathyroid surgery. Nevertheless, visual identification of the recurrent laryngeal nerve (RLN) remains the gold standard. Even if intermittent ioNM does not reduce the rate of postoperative RLN palsy, it enhances identification of the RLN and predicts in about 2/3 of the cases the findings of postoperative laryngoscopy (1). After its introduction in thyroid surgery, the concept of two-stage thyroidectomy in case of loss of signal (LOS) after dissection of the RLN on the first side has been proposed and strongly recommended at least in selected cases to avoid bilateral RLN palsy (2,3).
Continuous ioNM (C-ioNM), considered as the next step in nerve monitoring, provides real-time RLN evaluation during surgery and may help to detect impending injury of the RLN earlier than intermittent electromyographic (EMG) monitoring. Currently, promising data are available advising fewer permanent vocal fold palsies after thyroid surgery using C-ioNM compared with I-ioNM (4). However, all published data regarding C-ioNM in thyroid surgery refer to conventional open surgery, which allows an easy application of the vagal electrode and the electrode wires that can easily be inserted through the primary incision (5-8).
The use of C-ioNM during video-assisted thyroidectomy is barely described because of the limitations due to the narrow space available for the application and correct placement of the vagal electrode.
As a referral center in endocrine surgery with a large experience in the field of minimally invasive thyroid surgery we report on our first experiences with the C-ioNM during minimally invasive video-assisted thyroidectomy (MIVAT).
Methods
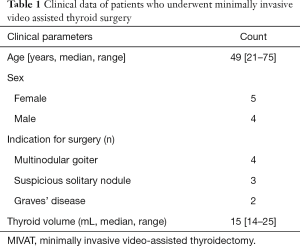
To evaluate the feasibility and applicability of C-ioNM in MIVAT we identified 9 patients. Preoperative clinical data was summarized in Table 1.
Full table
Written informed consent was obtained and the research was conducted according to the principles of the Declaration of Helsinki.
Operative technique
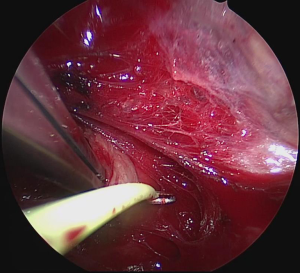
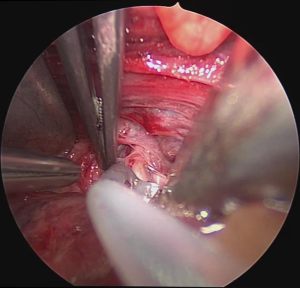
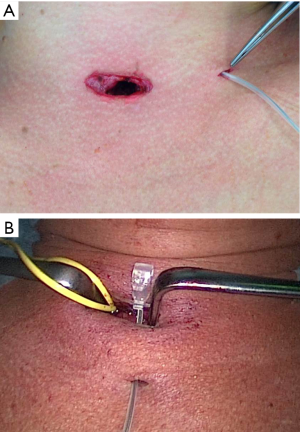
Surgery was performed on the basis of the technique first described by Miccoli (9). After a 2–4 cm skin incision above the sternal notch, the cervical linea alba was opened and the thyroid separated from the strap muscles. The surgical field was maintained using conventional retractors. The muscles were retracted laterally while the thyroid lobe was retracted medially. Further dissection was performed under video-assistance using a 5 mm 30° camera (Karl Storz GmbH®, Tuttlingen, Germany), leading to a 5-fold magnification of the structures and special instrumentation including slim scissors, spatula and a spatula shaped aspirator. Haemostasis was achieved with bipolar devices or ligature. The first step was visual identification of the vagus nerve and stimulation with a bipolar handheld stimulation probe (4 Hz, 200 µs, 1 mA; Dr. Langer Medical GmbH®, Waldkirch, Germany). The vagus nerve was separated from the surrounding tissue and a vessel loop was wrapped around the nerve (Figure 1). The electrode wires were either pulled through an additional skin incision on the dominating side lateral to the sternocleidomastoid muscle or through an additional incision in the midline below the cervical approach (Figure 2A,B). Then the saxophone-shaped single-channel electrode (3 Hz, 200 µs, 1 mA Dr. Langer Medical®, Waldkirch, Germany) was placed (Figure 3).
After correct placement of the electrode and baseline EMG recording, surgery continued with the dissection of the upper pole vessels as usual. To expose and dissect the upper pole vessels the thyroid lobe was retracted downward. After transection of the upper pole vessels the lobe was extracted through the primary skin incision and the RLN and parathyroid glands were identified and preserved. The dissection of the lobe was then completed under direct vision.
General anesthesia was maintained by complete intravenous application of Propofol (6–12 mg/kg/h) in combination with remifentanyl (0.1 µg/kg/min). No neuromuscular blocking agents were allowed during surgery.
EMG amplitude and latency changes were recorded especially with regard to potential reduction of the EMG amplitude >50% and/or increase in latency by >10% which was defined as LOS resulting in possible RLN damage (10).
Six patients were scheduled for total thyroidectomy because of Graves’ disease (n=2) or multinodular goiter (n=4) and three for lobectomy due to a suspicious solitary thyroid nodule. All patients underwent pre- and postoperative laryngoscopy. Preoperative characteristic of the patients, intraoperative data and postoperative complications were collected prospectively. Patients were generally discharged on the second postoperative day.
Results
All thyroid operations were completed as planned. The mean operating time was 98±13 min for lobectomy and 93±13 min for total thyroidectomy, including the electrode placement time.
In 6 patients, the electrode-wires were pulled through the additional skin incision on the dominating side lateral to the sternocleidomastoid muscle. In two patients, the wires were pulled through the additional incision in the midline below the surgical approach, and in one patient, the wires were diverted directly through the primary incision.
There were no complications during the placement of the electrode wires via the additional access. After a short learning curve the median time until correct placement of the vagal electrode was 5 minutes (range, 5–20 minutes).
Replacement of the electrode during surgery was not necessary, however in one patient with the electrode wires diverted through the primary incision we observed a dislocation of the vagus electrode during surgery and changed to intermittent nerve monitoring.
Intermittent EMG amplitude decrease >50% and increase of latency >10% was observed in one patient during the mobilization of the thyroid lobe. However, postoperative rate of RLN palsy was zero.
Discussion
Whereas almost all data published regarding C-ioNM in neck surgery refer to open surgery with large skin incisions, we were able to proof feasibility and applicability of C-ioNM in MIVAT. This study describes the use of C-ioNM during MIVAT with an additional lateral small skin incision for the first time.
The minimally invasive approach in thyroid surgery leads to an optimized cosmetic result and has gained worldwide acceptance. Two major limitations for C-ioNM during MIVAT have to be mentioned. The correct positioning of the vagal electrode through the small skin incision under video assistance is demanding due to the narrow working space. The diversion of the electrode wires through the primary skin incision leads to a high rate of electrode dislocation during the procedure especially when pulling the thyroid lobe through the small skin incision.
In contrast to another technique described, in which the electrode wires are diverted either directly through the primary skin incision through and between the thyroid lobe medially and the strap muscles laterally or a modified way through the primary skin incision between the space of the sternothyroid and sternocleidomastoid muscle (11), we investigated the ability to pull the electrode wires through an additional lateral skin incision. This maneuver turned out to be feasible, helpful and safe to avoid electrode dislocation during surgery. The only case of electrode dislocation we observed in this series was the case with the electrode wires diverted through the primary incision.
Traction is reported to be the most common cause of RLN palsy (12,13) and C-ioNM has the potential advantage to recognize threatening nerve lesions by monitoring nerve function nearly in real time.
Published data have shown that impending nerve injury can be recognized by EMG changes as combined events affecting amplitude and latency (10,14). Interestingly, several study groups report on amplitude variations and reduction up to 70% between animals and between the two nerves in the same animal, even if traction force was kept constant (15,16).
In our study we observed EMG amplitude changes of >50% and changes in latency of >10% in only one patient during the extraction of the thyroid lobe, which did not affect the surgical strategy and did not lead to a RLN palsy.
The rate of RLN injuries in specialized endocrine centers is low and the use of intermittent ioNM is not able to additionally reduce the incidence of RLN palsy. However, the use of ioNM allows inexperienced surgeons to perform a safe operation with a complication rate comparable to that obtained under supervision of an experienced surgeon (17) and ioNM is reported to be useful in challenging redo surgery in the neck (18).
Instead, the data published on C-ioNM seems to show that its use is associated with a significant lower permanent RLN palsy rate (4). If this data will be confirmed in further studies, C-ioNM will become undeniable in thyroid surgery also if performed by a minimally invasive approach.
Conclusions
In this study we showed that C-ioNM is feasible and safe during MIVAT. An additional skin incision to pull through the electrode wires is necessary to avoid electrode dislocation during surgery. Traction of the thyroid lobe after transection of the upper pole vessels during mobilization does not seem to affect RLN function.
However, more data have to be collected to definitely estimate significance of C-ioNM in minimally invasive thyroid surgery.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.05.17). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained and the research was conducted according to the principles of the Declaration of Helsinki (as revised in 2013). No concerns were raised from the ethical point of view (no approval number available).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Malik R, Linos D. Intraoperative Neuromonitoring in Thyroid Surgery: A Systematic Review. World J Surg 2016;40:2051-8. [Crossref] [PubMed]
- Dralle H, Lorenz K, Schabram P, et al. Intraoperative neuromonitoring in thyroid surgery. Recommendations of the Surgical Working Group for Endocrinology. Chirurg 2013;84:1049-56. [Crossref] [PubMed]
- Randolph GW, Dralle HInternational Intraoperative Monitoring Study Group, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 2011;121:S1-16. [Crossref] [PubMed]
- Schneider R, Sekulla C, Machens A, et al. Postoperative vocal fold palsy in patients undergoing thyroid surgery with continuous or intermittent nerve monitoring. Br J Surg 2015;102:1380-7. [Crossref] [PubMed]
- Schneider R, Lamade W, Hermann M, et al. Continuous intraoperative neuromonitoring of the recurrent laryngeal nerve in thyroid surgery (CIONM) - Where are we now? An update to the European Symposium of Continuous Neuromonitoring in Thyroid Surgery. Zentralbl Chir 2012;137:88-90. [Crossref] [PubMed]
- Schneider R, Machens A, Bucher M, et al. Continuous intraoperative monitoring of vagus and recurrent laryngeal nerve function in patients with advanced atrioventricular block. Langenbecks Arch Surg 2016;401:551-6. [Crossref] [PubMed]
- Mangano A, Kim HY, Wu CW, et al. Continuous intraoperative neuromonitoring in thyroid surgery: Safety analysis of 400 consecutive electrode probe placements with standardized procedures. Head Neck 2016;38:E1568-74. [Crossref] [PubMed]
- Bacuzzi A, Dralle H, Randolph GW, et al. Safety of Continuous Intraoperative Neuromonitoring (C-IONM) in Thyroid Surgery. World J Surg 2016;40:768-9. [Crossref] [PubMed]
- Miccoli P, Berti P, Conte M, et al. Minimally invasive surgery for thyroid small nodules: Preliminary report. J Endocrinol Invest 1999;22:849-51. [Crossref] [PubMed]
- Schneider R, Randolph GW, Sekulla C, et al. Continuous intraoperative vagus nerve stimulation for identification of imminent recurrent laryngeal nerve injury. Head Neck 2013;35:1591-8. [Crossref] [PubMed]
- Dionigi G, Chiang FY, Hui S, et al. Continuous Intraoperative Neuromonitoring (C-IONM) Technique with the Automatic Periodic Stimulating (APS) Accessory for Conventional and Endoscopic Thyroid Surgery. Surg Technol Int 2015;26:101-14. [PubMed]
- Chiang FY, Lu IC, Kuo WR, et al. The mechanism of recurrent laryngeal nerve injury during thyroid surgery--the application of intraoperative neuromonitoring. Surgery 2008;143:743-9. [Crossref] [PubMed]
- Wu CW, Dionigi G, Sun H, et al. Intraoperative neuromonitoring for the early detection and prevention of RLN traction injury in thyroid surgery: a porcine model. Surgery 2014;155:329-39. [Crossref] [PubMed]
- Phelan E, Schneider R, Lorenz K, et al. Continuous vagal IONM prevents recurrent laryngeal nerve paralysis by revealing initial EMG changes of impending neuropraxic injury: a prospective, multicenter study. Laryngoscope 2014;124:1498-505. [Crossref] [PubMed]
- Brauckhoff K, Aas T, Biermann M, et al. EMG changes during continuous intraoperative neuromonitoring with sustained recurrent laryngeal nerve traction in a porcine model. Langenbecks Arch Surg 2017;402:675-81. [Crossref] [PubMed]
- Béchu M, Lauzana E, Köhler P, et al. Inter- and intraindividual differences of vulnarability of recurrent laryngeal nerves under tensile stress in a porcine model. J Neurol Sci 2015;357:e245-6. [Crossref]
- Alesina PF, Hinrichs J, Meier B, et al. Intraoperative neuromonitoring for surgical training in thyroid surgery: its routine use allows a safe operation instead of lack of experienced mentoring. World J Surg 2014;38:592-8. [Crossref] [PubMed]
- Brauckhoff K, Vik R, Sandvik L, et al. Impact of EMG Changes in Continuous Vagal Nerve Monitoring in High-Risk Endocrine Neck Surgery. World J Surg 2016;40:672-80. [Crossref] [PubMed]