
The incidence of second primary malignancies after gastrointestinal stromal tumor before and after the introduction of imatinib mesylate
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract with an annual frequency of 10 to 14.5 per one million of the population (1). GISTs express the cell-surface transmembrane receptor c-kit, a protein coded by the KIT proto-oncogene possessing tyrosine kinase activity. The numerous mutations of KIT seen in GIST result in constitutive activation of tyrosine kinase signaling, leading to uncontrolled cell proliferation and resistance to apoptosis (2-4). Tumors that lack KIT mutations have been found to express activating mutations in the related tyrosine kinase platelet derived growth factor receptor alpha (PDGFR) (5,6). Diagnosis of GIST has greatly increased following pathologic reclassification and the widespread adoption of c-kit immunoshistochemical staining (7,8). Prior to 2000, many GISTs were misdiagnosed as other smooth muscle tumors including sarcoma and leiomyosarcoma (3,7).
Imatinib mesylate, a tyrosine kinase inhibitor, competitively inhibits KIT, BCR-ABL, ARG, PDGFR, and PDGFR tyrosine kinases (9-12). Imatinib was FDA approved in 2002 for the treatment of unresectable and metastatic GISTs, and has since become the standard of care. Its use has resulted in greatly improved survival rates (13,14). Historically, treatment of GISTs had consisted of surgical resection of localized disease with an overall 5 year survival rate of approximately 50% (15-17). Patients with more advanced disease that could not be resected had a median survival less than 21 months. Responses to conventional chemotherapy and/or radiotherapy were poor (16,18-20).
Improved longevity in patients with GIST raises questions regarding the development of second malignancies in these patients. Not only may these patients have an increased risk due to the presence of a primary malignancy, but imatinib itself has been implicated in the development of second primary malignancies following increased survival (21). Studies have demonstrated a small risk of second cancers in patients receiving therapy with tyrosine kinase inhibitors for hematologic malignancies, mostly for CML (22). Additionally, patients with GIST have also been shown to be at risk for the development of SPMs regardless of treatment (23-25). The actual risk, particularly with regard to use of imatinib, is unknown.
The purpose of this study was to examine the incidence of SPMs after GIST, particularly before (pre-imatinib era: 1992-2001) and after (imatinib era: 2002-2009), and factors related to the occurrence of SPMs using a population-based approach.
Methods
Data collection
Data from the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) 1992-2009 program were utilized. Registries included were those from the SEERS 13 (San Francisco-Oakland, Connecticut, Hawaii, Iowa, New Mexico, Seattle, Utah, Atlanta, San Jose-Monterrey, Louisiana, Alaska, rural Georgia, and Detroit), representing approximately 13.4% of the U.S. population (26). All cases examined were confirmed to be malignant microscopically, not by death certificate or autopsy. Patients included were only those with active follow-up with primary endpoint data. Cases excluded were those in which the primary site of the tumor was unknown, and those in which GIST was considered localized as these patients would not have been considered as candidates for imatinib therapy during the time period studied [imatinib was only recently approved for adjunctive therapy for localized surgical resection (27,28)].
Diagnostic codes used for data from 1992-2000 were 8936 (GIST) from any site, and 8935 (sarcoma), 8890 (leiomyosarcoma), and 9560 (neurilemmoma) in the gastrointestinal tract (middle 1/3 of esophagus until the rectum). We included these soft tissue tumors of the gastrointestinal tract as these were likely originally misclassified cases of GIST (3,29). As the diagnostic accuracy of GIST improved after the widespread use of c-kit staining, only tumors classified as GIST were examined from 2001-2009. Variables examined in our analysis were sex, race, marital status, radiation, grade, vital status, age of diagnosis, months of survival, and person-time-years (time during which a subject is at risk of the study event).
Statistical analysis
The observed incidence of SPMs after GISTs was determined over time, as well as in each of the time periods, pre-imatinib and imatinib. Standardized incidence ratios (SIRs) and 95% confidence intervals (CIs) were calculated using the estimated incidence in the age-adjusted general population in each of the time periods using SEER*Stat 8.0.1. Observed incidences were then compared between pre- and post-imatinib eras using Fisher’s exact test.
The relationship between the presence of SPMs and each of the variables was examined using Fisher’s exact test for categorical variables and Wilcoxon rank-sum test for continuous variables. Variables found to be significant or marginally significant (P<0.10) in each of the analyses were included in a logistic regression analysis that was then used to examine the odds of having an SPM or not. A similar analysis was undertaken to examine the relationship of era (pre- or post-imatinib) to each of the variables. Survival analysis was done using Kaplan-Meier method. Non-parametric measures were utilized due to the low incidence of SPMs. Statistical analysis system (SAS) was used for analysis. For all values, the significance level was set to P<0.05.
Results
Overall, the rate of SPMs after GIST in the imatinib era was 7.07%, compared with the rate of 1.15% that occurred in the pre-imatinib era (P=0.030). This difference was mainly accounted for by a higher incidence of colon adenocarcinoma in the imatinib era (P=0.023). Renal cell carcinoma also accounted for this difference. In the imatinib era, the SIR of renal cell carcinoma was 4.57, which was significantly elevated compared with the expected age- and time- adjusted incidence for the general population (95% CI: 1.68-9.96). In contrast, the rate of melanoma of the skin was significantly lower in the imatinib era compared with the pre-imatinib era (P=0.030). In the pre-imatinib era for melanoma, the SIR was 17.64 (95% CI: 3.64-51.57) (Table 1).
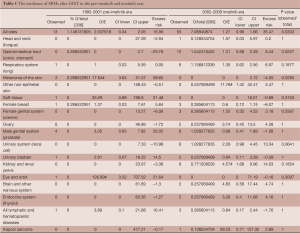
Full table
Patients with SPMs were often older at diagnosis (mean =64.18, SD =12.95) than patients without SPMs (mean =60.63, SD =15.27, P=0.024) (Figure 1). Marital status was significantly related to the presence of SPMs (P=0.0154). There were more married patients with SPMs (78.26%) than without SPMs (65.62%).
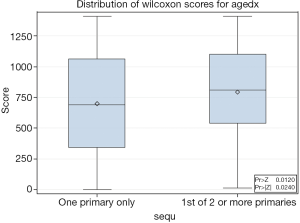
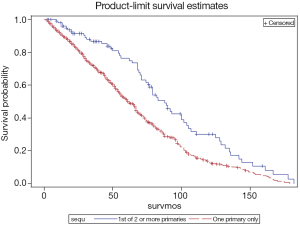
There was no significant difference in person-time years. Patients with SPMs were at risk for 5 years (SD =5.32), while patients without were at risk for 2.93 years (SD =2.79). Sex (P=1.00), race (P=0.3631), grade (P=0.6862), radiation treatment (P=1.00) were not associated with the presence of SPMs (Table 2). In the multivariable logistic regression analysis, age was the most important factor related to someone’s odds of developing an SPM or not in any time period. Patients who were older had a 3.7% greater odds per year (OR =1.037, CI: 1.002-1.073) of developing an SPM. Of note, patients with SPMs were more likely to be alive (62.5%) than those without SPMs (45.68%, P=0.0010) at the end of follow-up. In addition, they had greater number of months of survival (mean =70.83, SD =51.54) than those without SPMs (mean =39.33, SD =37.30, P<0.0001) (Figure 2).
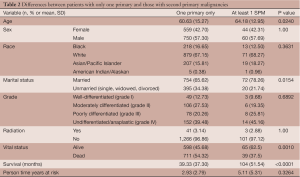
Full table
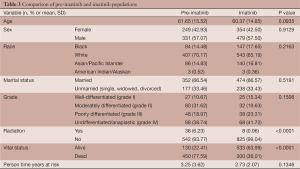
For validation of the pre-imatinib and imatinib era comparisons, other factors were compared between these cases. There were no differences between the pre-imatinib and imatinib eras with regard to age (P=0.0937), sex (P=0.9129), race (P=0.2163), marital status (P=1.00), grade (P=0.1506), or person time years (P=0.1346). There were more patients in the post-imatinib era alive (n=533, 63.99%) than in the pre-imatinib era (n=130, 22.41%) by the end of follow-up (P<0.0001). There were more people in the pre-imatinib era who received radiation for their tumors (n=36, 6.23%) than in the imatinib era (n=8, 0.96%) (Table 3).
Full table
Discussion
Our results demonstrate a higher incidence of certain SPMs after GIST compared with the general population, particularly melanoma and renal cancers (Table 1). This is consistent with previous studies which demonstrate the development of SPMs following increased survival after GIST (21,23). The higher incidence may also be related to increased medical surveillance following primary diagnosis, exposure to risk factors for GIST, or genetic predispositions of individuals to cancer. A small percentage of GISTs (less than 5%) may be associated with autosomal dominant germ line Kit or PDGFR mutations (30), which may predispose patients to develop tumor syndromes such as neurofibromatosis type 1, Carney triad, and familial GIST syndrome (31). There have been several reviews and case reports that demonstrate that GIST may occur synchronously with other tumors (23-25,31-35). These may be a result of a common exposure to carcinogenic agents resulting in the concurrent presence of malignancies. A study of 783 patients with GIST showed that approximately 20% develop other primary malignancies (23). The most common malignancies reported in patients with GIST include hematologic, prostate, breast, kidney, lung, female genital tract, and carcinoid tumors. Soft tissue and bone sarcomas, malignant melanoma, and seminoma have also been reported after GIST (24). Acute myelogenous leukemia has also been thought to be associated with GIST (36). Our findings of significantly higher rates of melanoma and genitourinary cancers, particularly renal cell carcinoma, after GIST are in line with these. Renal cancers occurred at a disproportionately higher rate than that for the general population after the introduction of imatinib, while melanoma occurred at lower rates after the introduction of imatinib.
While most melanomas involve persistent activation of MAPK pathways that involve signaling through serine/threonine kinase BRAF, various growth factor receptors including c-kit are likely overactivated in this cascade (37). A small percentage of melanomas demonstrate activating mutations of KIT, for which imatinib demonstrates significant efficacy (38,39). The observed decrease in incidence of melanoma in patients with GIST after the introduction of imatinib may speak to this shared mechanism by which GIST and melanoma evolve. The relationship between imatinib and renal cancers is less clear. There is a well-demonstrated role for vascular endothelial growth factor (VEGF) receptor tyrosine kinases in the pathogenesis of renal cell carcinoma (40). Tyrokine kinase inhibitors that target VEGF such as sunitinib have been successfully used in the treatment of renal cell carcinoma (41). Sunitinib is a distinct class of tyrosine kinase inhibitor with an entirely different mechanism than imatinib. It is unlikely to have affected a decrease in the incidence of renal cancers in GIST patients, but it remains unclear as to why the incidence would have risen. This is also the case for second primary gastrointestinal cancers (mostly colon adenocarcinomas), which occurred at a higher rate in GIST patients after the introduction of imatinib in our study. This, in addition to the higher rate of genitourinary cancers in patients with GIST, is consistent with findings in the literature (23,24). VEGF also plays a role in the pathogenesis of colon cancer, in addition to epidermal growth factor receptor (EGFR). Agents targeting VEGF and EGFR are utilized in colon cancer (42,43), which also have distinct targets from imatinib.
In our sub-analysis of the risk factors for SPMs after GIST, we found that older and married patients are more likely to develop SPMs. This is likely related to their increased survival and time available to develop SPMs. We found that patients who went on to develop SPMs had more months of survival and were more likely to be alive at the end of follow up. Several studies have shown that marriage is associated with increased survival (44-46). This finding, however, does not downplay the role of other factors such as imatinib in the increased incidence of SPMs. As our findings show, person-time-years was not significantly different between patients between the 2 eras (Table 3), implying that survival time was not the only risk factor for the development of SPMs.
The biggest limitation to our study is the assumption that imatinib was offered to patients who met the criteria for treatment after its FDA approval as SEER does not collect data on medication. To support this assumption, we demonstrated that there were no significant differences between the pre- and post-imatinib population with regard to age, sex, marital status, or grade (Table 3). There was however a significant difference with regard to the administration of radiation, a treatment modality that is recorded by SEER. As radiation was shown to be ineffective, it was used significantly less frequently in the era of imatinib. This further supports the ability of SEER data to detect patterns in treatment modalities. Another limitation was that GISTs were not able to be distinguished from other gastrointestinal smooth muscle tumors prior to widespread use of c-kit staining, and were often misclassified. We corrected for this by identifying and including sarcomas, leiomyosarcomas and neurilemomas as tumors for which early GISTs were likely mistaken (3,29). As in many epidemiologic survey studies, we must also be aware of the surveillance bias, which may have affected the incidence of SPMs in patients who already carried a primary diagnosis of cancer.
In summary, the findings in our study demonstrate that patients after GIST are at increased risk of developing SPMs and that this risk is increased following the introduction of imatinib in 2002, particularly those of the gastrointestinal and genitourinary tracts. While it is unknown why there is an increased risk of these cancers, the increased incidence of SPM in the era of imatinib is likely explained by the increased survival of patients with metastatic GIST and therefore more time available to develop SPM. Nonetheless, clinicians following these patients should certainly be aware of the risk to allow for proper follow-up. Further studies are needed to investigate the mechanism.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2013.07.04). MX serves as an unpaid editorial board member of Translational Cancer Research. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki. The study was reviewed and determined by the IRB to be exempt from formal committee review as it was research involving the collection of data from a source that was publicly available and did not contain unique patient identifiers. Informed consent was not obtained as it was a SEER database epidemiology study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nilsson B, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer 2005;103:821-9. [PubMed]
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577-80. [PubMed]
- Rubin BP, Fletcher JA, Fletcher CD. Molecular insights into the histogenesis and pathogenesis of gastrointestinal stromal tumors. Int J Surg Pathol 2000;8:5-10. [PubMed]
- Ali S, Ali S. Role of c-kit/SCF in cause and treatment of gastrointestinal stromal tumors (GIST). Gene 2007;401:38-45. [PubMed]
- Corless CL, Schroeder A, Griffith D, et al. PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol 2005;23:5357-64. [PubMed]
- Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 2003;21:4342-9. [PubMed]
- Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol 1999;30:1213-20. [PubMed]
- Rubin BP, Singer S, Tsao C, et al. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res 2001;61:8118-21. [PubMed]
- Buchdunger E, Cioffi CL, Law N, et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther 2000;295:139-45. [PubMed]
- Heinrich MC, Griffith DJ, Druker BJ, et al. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood 2000;96:925-32. [PubMed]
- Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med 1996;2:561-6. [PubMed]
- Okuda K, Weisberg E, Gilliland DG, et al. ARG tyrosine kinase activity is inhibited by STI571. Blood 2001;97:2440-8. [PubMed]
- Perez EA, Livingstone AS, Franceschi D, et al. Current incidence and outcomes of gastrointestinal mesenchymal tumors including gastrointestinal stromal tumors. J Am Coll Surg 2006;202:623-9. [PubMed]
- Artinyan A, Kim J, Soriano P, et al. Metastatic gastrointestinal stromal tumors in the era of imatinib: improved survival and elimination of socioeconomic survival disparities. Cancer Epidemiol Biomarkers Prev 2008;17:2194-201. [PubMed]
- Clary BM, DeMatteo RP, Lewis JJ, et al. Gastrointestinal stromal tumors and leiomyosarcoma of the abdomen and retroperitoneum: a clinical comparison. Ann Surg Oncol 2001;8:290-9. [PubMed]
- DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51-8. [PubMed]
- Ng EH, Pollock RE, Romsdahl MM. Prognostic implications of patterns of failure for gastrointestinal leiomyosarcomas. Cancer 1992;69:1334-41. [PubMed]
- Licht JD, Weissmann LB, Antman K. Gastrointestinal sarcomas. Semin Oncol 1988;15:181-8. [PubMed]
- Zalupski M, Metch B, Balcerzak S, et al. Phase III comparison of doxorubicin and dacarbazine given by bolus versus infusion in patients with soft-tissue sarcomas: a Southwest Oncology Group study. J Natl Cancer Inst 1991;83:926-32. [PubMed]
- Shiu MH, Farr GH, Papachristou DN, et al. Myosarcomas of the stomach: natural history, prognostic factors and management. Cancer 1982;49:177-87. [PubMed]
- Roy L, Guilhot J, Martineau G, et al. Unexpected occurrence of second malignancies in patients treated with interferon followed by imatinib mesylate for chronic myelogenous leukemia. Leukemia 2005;19:1689-92. [PubMed]
- Verma D, Kantarjian H, Strom SS, et al. Malignancies occurring during therapy with tyrosine kinase inhibitors (TKIs) for chronic myeloid leukemia (CML) and other hematologic malignancies. Blood 2011;118:4353-8. [PubMed]
- Pandurengan RK, Dumont AG, Araujo DM, et al. Survival of patients with multiple primary malignancies: a study of 783 patients with gastrointestinal stromal tumor. Ann Oncol 2010;21:2107-11. [PubMed]
- Agaimy A, Wünsch PH, Sobin LH, et al. Occurrence of other malignancies in patients with gastrointestinal stromal tumors. Semin Diagn Pathol 2006;23:120-9. [PubMed]
- Wronski M, Ziarkiewicz-Wroblewska B, Gornicka B, et al. Synchronous occurrence of gastrointestinal stromal tumors and other primary gastrointestinal neoplasms. World J Gastroenterol 2006;12:5360-2. [PubMed]
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 13 Regs Research Data, Nov 2012 Sub (1992-2010) <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, based on the November 2012 submission.
- Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:1097-104. [PubMed]
- Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 2012;307:1265-72. [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 2006;130:1466-78. [PubMed]
- Gupta P, Tewari M, Shukla HS. Gastrointestinal stromal tumor. Surg Oncol 2008;17:129-38. [PubMed]
- Ruka W, Rutkowski P, Nowecki Z, et al. Other malignant neoplasms in patients with gastrointestinal stromal tumors (GIST). Med Sci Monit 2004;10:LE13-4. [PubMed]
- Liu YJ, Yang Z, Hao LS, et al. Synchronous incidental gastrointestinal stromal and epithelial malignant tumors. World J Gastroenterol 2009;15:2027-31. [PubMed]
- Liszka Ł, Zielińska-Pajak E, Pajak J, et al. Coexistence of gastrointestinal stromal tumors with other neoplasms. J Gastroenterol 2007;42:641-9. [PubMed]
- Kawanowa K, Sakuma Y, Sakurai S, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol 2006;37:1527-35. [PubMed]
- Ponti G, Luppi G, Martorana D, et al. Gastrointestinal stromal tumor and other primary metachronous or synchronous neoplasms as a suspicion criterion for syndromic setting. Oncol Rep 2010;23:437-44. [PubMed]
- Miettinen M, Kraszewska E, Sobin LH, et al. A nonrandom association between gastrointestinal stromal tumors and myeloid leukemia. Cancer 2008;112:645-9. [PubMed]
- Monsel G, Ortonne N, Bagot M, et al. c-Kit mutants require hypoxia-inducible factor 1alpha to transform melanocytes. Oncogene 2010;29:227-36. [PubMed]
- Carvajal RD, Antonescu CR, Wolchok JD, et al. KIT as a therapeutic target in metastatic melanoma. JAMA 2011;305:2327-34. [PubMed]
- Guo J, Si L, Kong Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol 2011;29:2904-9. [PubMed]
- Nicol D, Hii SI, Walsh M, et al. Vascular endothelial growth factor expression is increased in renal cell carcinoma. J Urol 1997;157:1482-6. [PubMed]
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:3584-90. [PubMed]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [PubMed]
- Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med 2007;357:2040-8. [PubMed]
- Berntsen KN. Trends in total and cause-specific mortality by marital status among elderly Norwegian men and women. BMC Public Health 2011;11:537. [PubMed]
- Manzoli L, Villari P. Marital status and mortality in the elderly: a systematic review and meta-analysis. Soc Sci Med 2007;64:77-94. [PubMed]
- Johnson NJ, Backlund E, Sorlie PD, et al. Marital status and mortality: the national longitudinal mortality study. Ann Epidemiol 2000;10:224-38. [PubMed]


