
Outcomes of patients with large cell neuroendocrine carcinoma of the lung after complete resection
IntroductionOther Section
Tumors of the lung with neuroendocrine morphology comprise a spectrum of tumor types with distinct biology and clinical features; they include low-grade typical carcinoid, intermediate-grade atypical carcinoid, high-grade large cell neuroendocrine carcinoma (LCNEC), and small cell lung cancer (SCLC) according to the recent revision of the World Health Organization (WHO) classification of lung and pleural tumors (1). LCNEC fulfilled the following criteria: neuroendocrine morphology, mitotic rate greater than 10 per 10 high-power field, necrosis (often large zones), cytologic features of non-small cell lung cancer (NSCLC), and positive immunohistochemical staining for one or more neuroendocrine markers (2). Among high-grade neuroendocrine carcinomas, LCNEC is rarer than SCLC (1,3). Most data related to the treatment of LCNEC is based on retrospective analyses of small case series (4,5). Thus, the surgical outcomes of LCNEC patients and optimal treatment approaches are not well known. For instance, it is unclear whether LCNEC should be treated chemotherapeutically as a SCLC or as a NSCLC. To address this issue, the present study evaluated the prognosis of patients with surgically-treated LCNEC.
MethodsOther Section
Patients demographics
Between January 2008 and December 2014, a total of 17,184 patients underwent surgical resection of primary lung cancer (NSCLC) at the Department of Thoracic Surgery of Shanghai Chest Hospital (Shanghai 200030, China). Of these patients, we reviewed the surgical records of 104 (0.6%) consecutive cases treated by tumor resection for primary LCNEC, which was determined based on the diagnostic criteria proposed by the 2015 edition of the WHO classification system (1). All patients underwent a complete preoperative examination and were considered as having tumors that were potentially curable by surgical resection. Regarding combined LCNEC, immunohistochemistry staining was evaluated for each C-LCNEC and non-LCNEC component and was regarded as positive if at least 10% of the tumor cells were stained. Patients with combined LCNEC, local macroscopic/microscopic neoplastic residue, sublobar resections or lymph node sampling were excluded. There was no perioperative death. Data for the remaining 90 patients who underwent complete tumor resection and lymphadenectomy were analyzed. The study protocol was approved by the institutional review board.
Preoperative work-up
All patients underwent a preoperative examination to exclude distant metastasis which include computed tomography scan of the chest; adrenal gland, liver, and brain (subsequent magnetic resonance imaging was performed while brain metastases were suspected); bone scanning; ultrasonography examination; fibro bronchoscopy; and biopsy (endo/transbronchial, transthoracic) when possible. At our institution, only patients with mediastinal lymph node enlargement (>l cm) routinely underwent cervical mediastinoscopy or endobronchial ultrasound-guided transbronchial needle aspiration or were examined by positron emission tomography to exclude mediastinal lymphatic metastasis. Disease stage was determined based on the tumor-node-metastasis (TNM) classification using the International Union against cancer staging system (6). Clinical data including sex, age, smoking history, performance status, location (central/peripheral), type of resection, pathological stage, and post-operative adjuvant regimens were collected.
Follow-up
All patients with tolerable status were suggested to receive adjuvant chemotherapy at the first month after surgery. Patients were followed-up every 3 months for the first year and then every 3 to 6 months thereafter. Disease-free survival (DFS) was calculated from the day of operation to the time of first relapse (recurrence or metastasis). Overall survival (OS) was calculated from the day of operation to the date of death from any cause or of the last follow-up.
Statistical methods
Normally distributed continuous variables are presented as the mean ± standard deviation (SD), otherwise as the median and range, while categorical variables are presented as numbers and percentages. Survival rates were calculated with the Kaplan-Meier method. All patients who were alive at the last follow-up were censored. The log-rank test was used to compare survival between different groups. The Cox proportional hazard model was used to evaluate the effects of variables that could affect prognosis. All tests were two-sided and a P value <0.05 was considered to indicate statistical significance. Statistical analyses were performed using SPSS v.19.0 software (SPSS Inc., Chicago, IL, USA).
ResultsOther Section
Patient characteristics and treatment
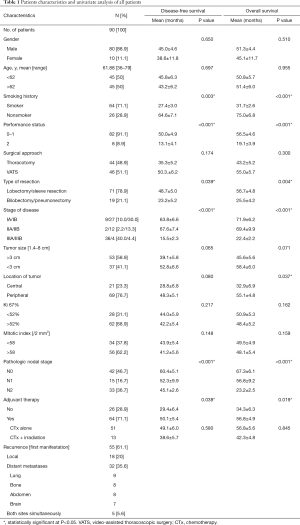
The demographic characteristics of the all patients are shown in Table 1. Of the 90 cases of LCNEC, 21 (23.3%) were central and 69 (76.7%) were peripheral tumors. A preoperative tissue diagnosis was made in 62 patients (68.9%); only a small fraction of these patients were diagnosed with LCNEC (n=9, 10%), but most were diagnosed with nonspecific cell types, including NSCLC (n=7, 7.8%), atypical carcinoid (n=6, 6.7%), and poorly differentiated carcinoma (n=8, 8.9%). Other biopsy specimens were manifested as necrotic lesions (punctate foci or large infarctlike zones).
Full table
The distribution of pathologic stage was stage IA in 9 patients (10%), stage IB in 27 (30%), stage IIA in 2 (2.2%), stage IIB in 12 (13.3%), and stage IIIA in 36 (40%), and stage IIIB in 4 patients (4.4%). The mean Ki-67 labeling index for all patients was 52.4% (range, 6.5–88.2%), and the mean mitotic index was 58/2 mm2 (range, 38–98/2 mm2). All 90 patients had tumor necrosis (median, 50%; range, 10–80%).
Adjuvant treatment
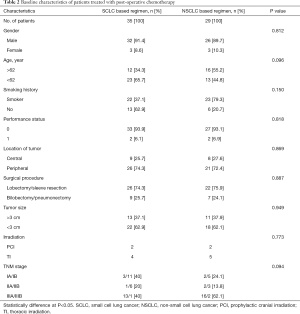
Sixty-four of 90 patients (71.1%) received post-operative chemotherapy (platinum-based regimen), including 32.8% (21/64) stage I diseases, 17.2% (11/64) stage II diseases, 50% (32/64) stage III diseases, respectively. The types of chemotherapeutic regimens were at the discretion of the treating physicians. Four cycles of chemotherapy were recommended as postoperative adjuvant therapy. All chemotherapy regimens were repeated every 3 weeks, up to 4 cycles or until the occurrence of patients’ intolerance. And these patients were treated with an SCLC-based regimen (etoposide/cisplatin; n=35) or an NSCLC-based regimen (gemcitabine, n=9; vinorelbine, n=8; pemetrexed, n=7; taxol, n=5). There was no significant difference in baseline characteristics between the SCLC-based and NSCLC-based regimen groups (Table 2). Besides, among the 64 patients, 5 patients with preoperatively diagnosed as N2 disease received induction chemotherapy, 4 with stage IIIb disease received prophylactic cranial irradiation, 9 with stage IIIa disease received adjuvant thoracic irradiation, respectively.
Full table
Of the 26 patients who did not receive adjuvant therapy, eight patients are due to relatively poor performance status. Six discontinued the treatment because of the intolerable side effects. Nine had poor treatment compliance due to fear of the potential side effects caused by chemotherapy. Three patients did not to receive chemotherapy because of poor economic status.
Survival analysis
The median follow-up was 35 months (range, 2.0–100 months). Within the follow-up period, 55 (61.1%) patients relapsed (Table 1) and 52 (57.8%) died. The 5-year DFS was 32.2% (49.5% for stage I, 59.7% for stage II, and 5.9% for stage III), whereas the 5-year OS was 36.3% (58.8% for stage I, 62.5% for stage II, and non-evaluated for stage III).
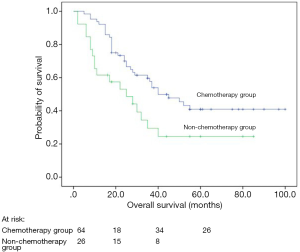
Univariate analyses revealed that post-operative chemotherapy (P=0.038), types of resection (P=0.039), smoking history (P=0.003), performance status (P<0.001), and TNM stage (P<0.001) were significant prognostic factors for DFS whereas post-operative chemotherapy (P=0.019; Figure 1), types of resection (P=0.004), tumor location (P=0.037), smoking history (P<0.001), performance status (P<0.001), and TNM stage (P<0.001) were significant prognostic factors for OS (Table 1).
A multivariate analysis for DFS showed that smoking history (no vs. yes, HR =0.463; 95% CI: 0.237–0.903; P=0.024), postoperative chemotherapy (yes vs. no, HR =0.278; 95% CI: 0.146–0.528; P<0.001), and early pathological stage (stage I vs. stage III: HR =0.106, 95% CI: 0.049–0.229; P<0.001; stage II vs. III, HR =0.097, 95% CI: 0.035–0.268, P<0.001) were independent prognostic factors of good outcome (Table 3). On the other hand, the multivariate Cox model indicated that smoking history (no vs. yes, HR=0.390; 95% CI: 0.182–0.835; P=0.015), pathological stage (stage I vs. stage III: HR =0.098, 95% CI: 0.043–0.221; P<0.001; stage II vs. III, HR =0.119, 95% CI: 0.044–0.324, P<0.001), and postoperative chemotherapy (yes vs. no, HR =0.223; 95% CI: 0.112–0.443; P<0.001) were significant prognostic factors for OS (Table 3).

Full table
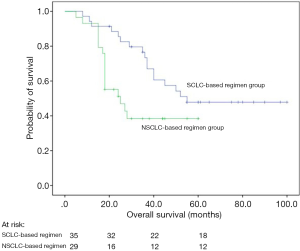
Both uni- and multivariate analyses showed that patients who received a SCLC-based chemotherapy regimen survived longer than those who received an NSCLC-based regimen (P=0.029; HR =0.420, 95% CI: 0.189–0.936, P=0.034 after adjustment; Figure 2, Table 4).

Full table
DiscussionOther Section
We retrospectively reviewed data on 90 patients with pulmonary LCNEC who underwent surgical resection. Long-term survival and DFS were influenced by smoking history, pathological stage, and post-operative chemotherapy.
In accordance with previous studies (7,8), patients with LCNEC were predominantly males and smokers. In our study, smoking history was a significant negative prognostic factor for OS and DFS, which further confirmed the link between tobacco and LCNEC.
LCNEC is a relatively uncommon tumor; the overall incidence of resectable LCNEC is estimated to be <3% (9-11). In our study, the incidence was 0.6% of all patients who underwent thoracic surgery. However, another study carried out in Japan found an incidence of LCNEC of 3.1% (7); thus, disease incidence may differ according to region.
LCNEC is difficult to identify preoperatively and is likely under-diagnosed, since biopsies are usually insufficient for correct diagnosis. In our study, the preoperative diagnosis in most patients was poorly differentiated carcinoma or NSCLC. There are several possible reasons for this finding. Firstly, LCNEC has the similar clinical and radiological features as other lung cancers, and is therefore difficult to distinguish solely on the basis of its presentation. Secondly, it is difficult to perform transbronchial biopsy on these largely peripheral tumors. Thirdly, a small biopsy or cytological specimen through percutaneous CT-guided biopsy may not be representative of the entire lesion (12). Fourthly, the morphology of LCNEC must be identified by light microscopy; other potential diagnoses includes SCLC, adenocarcinoma, and NSCLC, especially poorly differentiated adeno- or squamous cell carcinoma. Fifthly, the size difference between “small” and “large” cells is not absolute and can obscure diagnoses (13). Finally, since the material used for diagnosis (based on biopsy or cytology) is limited, and the typical morphology of neuroendocrine carcinoma cells is absent; it is therefore recommended to perform immunohistochemical staining for neuroendocrine markers (14).
The 5-year survival of LCNEC ranges from 13–57%; patients with LCNEC have worse survival than those with classic large-cell carcinoma or other NSCLCs (7). The 5-year OS of patients with LCNEC was 36.3% in our study, which is consistent with the rate of 35.5% reported by some investigators (7), but lower than the rate of 43% (8) and 40.3% (15) reported by others. This could be due to differences in the percentages of cases in each stage of the disease. In the present study, 36 patients (40%) were stage IIIA and four (4.4%) were stage IIIB. Among these patients, only five patients were preoperatively diagnosed with N2 disease and relieved after receiving induction chemotherapy. The other patients were unexpectedly detected after surgery.
LCNEC represents a subtype of high-grade neuroendocrine tumor and has poor prognosis similar to SCLC. Chemotherapy and radiotherapy are standard treatments for SCLC patients, even those with limited disease. However, whether LCNEC should be treated in a similar manner as SCLC was unknown. Because there was no significant difference between the chemotherapy alone group and chemoradiotherapy group for survival (P=0.845; Table 1). So we recognized them as the same group (post-operative chemotherapy group). In our study, 64 LCNEC patients received post-operative adjuvant treatment after complete resection and showed a significantly better prognosis than those not, which were similar to previous reports (16-18). However, Filosso et al. (19) analyzed 135 patients with LCNEC and found that postoperative adjuvant chemotherapy (platinum plus etoposide) was not a prognostic impact factor. However, they did not clarify the indication of adjuvant treatment after surgical resection. Perhaps most of the patients received postoperative chemotherapy in advanced pathological stage. Furthermore, in their study, the retrospective and multicenter design and the long recruitment period (over 17 years) may result in some bias. In recent years, the rate of complete resection and systematic nodal dissection has increased and the use of platinum-based adjuvant chemotherapy has become more systematic, which could explain the increased OS in our patients. The patients who did not receive post-operative adjuvant treatment were mainly due to their relatively poor physical conditions, intolerable or fear of the side effects. Previous reports showed that SCLC patients could benefit a lot from chemotherapy (20-22). Fournel et al. (23) reported that post-operative adjuvant chemotherapy was effective in stage I patients with LCNEC. We did not discuss this difference in our study, because of the very limited sample size and relatively insufficient follow-up time.
Due to the complex clinicopathological and biological features of patients with LCNEC, it remains unclear what constitutes an optimal chemotherapy regimen for LCNEC (14). Among others, Sun et al. insisted on treating LCNEC with SCLC treatment regime (24), while Varlotto et al. claimed that LCNEC should receive treatment similar to NSCLC (25). The database of Varlotto et al. used did not record the administration of chemotherapy, and therefore, it was a limitation of his study. In our study, there was a significant difference between SCLC- and NSCLC-based regimens in terms of the OS of patients. It was consistent with the findings of another study (26,27). In another report, 3-year and recurrence-free survival rates of 23 resected LCNEC cases treated with irinotecan plus cisplatin were 86% and 74%, respectively, indicating that this was an effective post-operative adjuvant chemotherapy regimen (28). There is currently an ongoing randomized phase III trial in Japan comparing irinotecan/cisplatin and standard etoposide/cisplatin adjuvant regimens in patients with completely resected LCNEC (29).
This study had some limitations. Firstly, it’s a retrospective study and only one institution was involved and the study population was relatively small. There have been no phase II or III trials for this rare tumor. A larger number of LCNEC patients should be studied prospectively in the future. Another limitation of the present study is the variety of drugs that were administered as treatment. A total of 35 patients received a standard SCLC-based regimen (etoposide/cisplatin) while others received NSCLC-based regimens (gemcitabine, vinorelbine, pemetrexed, or taxol). This variability may have differentially impacted LCNEC patient prognosis after completely resection.
ConclusionsOther Section
In conclusion, LCNEC is a rare, aggressive, and preoperatively difficult-to-diagnosis tumor that is associated with poor prognosis and high recurrence rates. Nevertheless, the results of this study suggest that LCNEC patients should receive adjuvant chemotherapy following surgical resection, preferentially with an SCLC-based chemotherapy regimen.
AcknowledgmentsOther Section
Funding: The authors would like to acknowledge support from the Grant No. 81572693 from the National Natural Science Foundation of China and Grant No. YG2015ZD14 from Medicine and Engineering Cross Foundation of Shanghai Jiaotong University.
FootnoteOther Section
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.06.14). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study has been approved by the ethics committee of Shanghai Chest Hospital (No. KS1707). Informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Chen LC, Travis WD, Krug LM. Pulmonary neuroendocrine tumors: what (little) do we know? J Natl Compr Canc Netw 2006;4:623-30. [Crossref] [PubMed]
- Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539-44. [Crossref] [PubMed]
- Travis WD, Linnoila RI, Tsokos MG, et al. Neuroendocrine tumours of the lung with proposed criteria for large cell neuroendocrine carcinoma. An ultrastructural, immunohis tochemical, and flow cytometric study of 35 cases. Am J Surg Pathol 1991;15:529-53. [Crossref] [PubMed]
- Dresler CM, Ritter JH, Patterson GA, et al. Clinicopathologic analysis of 40 patients with large cell neuroendocrine carcinoma of the lung. Ann Thorac Surg 1997;63:180-5. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Iyoda A, Hiroshima K, Toyozaki T, et al. Clinical characterization of pulmonary large cell neuroendocrine carcinoma and large cell carcinoma with neuroendocrine morphology. Cancer 2001;91:1992-2000. [Crossref] [PubMed]
- Veronesi G, Morandi U, Alloisio M, et al. Large cell neuroendocrine carcinoma of the lung: a retrospective analysis of 144surgical cases. Lung Cancer. 2006;53:111-5. [Crossref] [PubMed]
- Yeh YC, Chou TY. Pulmonary neuroendocrine tumors: study of 90 cases focusing on clinicopathological characteristics, immunophenotype, preoperative biopsy, and frozensection diagnoses. J Surg Oncol 2014;109:280-6. [Crossref] [PubMed]
- Paci M, Cavazza A, Annessi V, et al. Large cell neuroendocrine carcinoma of the lung: a 10-year clinic pathologic retrospective study. Ann Thorac Surg 2004;77:1163-7. [Crossref] [PubMed]
- Jiang SX, Kameya T, Shoji M, et al. Large cell neuroendocrine carcinoma of the lung: a histologic and immunohistochemical study of 22 cases. Am J Surg Pathol 1998;22:526-37. [Crossref] [PubMed]
- Travis WD, Muller-Hermelink HK, Harris CC, et al. Pathology and genetics of tumours of the lung, pleura thymus and heart. In: World Health Organization international histological classification of tumours. Lyon: IARC, 2004.
- Marchevsky AM, Gal AA, Shah S, et al. Morphometry confirms the presence of considerable nuclear size overlap between “small cells” and “largecells” in high grade pulmonary neuroendocrine neoplasms. Am J Clin Pathol 2001;116:466-72. [Crossref] [PubMed]
- Iyoda A, Makino T, Koezuka S, et al. Treatment options for patients with large cell neuroendocrine carcinoma of the lung. Gen Thorac Cardiovasc Surg 2014;62:351-6. [Crossref] [PubMed]
- Asamura H, Kameyo T, Matsuno Y, et al. Neuroendocrine neoplasms of the lung: a prognostic spectrum. J Clin Oncol 2006;24:70-6. [Crossref] [PubMed]
- Iyoda A, Hiroshima K, Moriya Y, et al. Prospective study of adjuvant chemotherapy for pulmonary large cell neuroendocrine carcinoma. Ann Thorac Surg 2006;82:1802-7. [Crossref] [PubMed]
- Saji H, Tsuboi M, Matsubayashi J, et al. Clinical response of large cell neuroendocrine carcinoma of the lung to perioperative adjuvant chemotherapy. Anticancer Drugs 2010;21:89-93. [Crossref] [PubMed]
- Abedallaa N, Tremblay L, Baey C, et al. Effect of chemotherapy in patients with resected smallcell or large-cell neuroendocrine carcinoma. J Thorac Oncol 2012;7:1179-83. [Crossref] [PubMed]
- Filosso PL, Rena O, Guerrera F, et al. Clinical management of atypical carcinoid and large-cell neuroendocrine carcinoma: a multicentre study on behalf of the European Association of Thoracic Surgeons (ESTS) Neuroendocrine Tumours of the Lung Working Group†. Eur J Cardiothorac Surg 2015;48:55-64. [Crossref] [PubMed]
- Corso CD, Rutter CE, Park HS, et al. Role of Chemoradiotherapy in Elderly Patients With Limited-Stage Small-Cell Lung Cancer. J Clin Oncol 2015;33:4240-6. [Crossref] [PubMed]
- Behera M, Ragin C, Kim S, et al. Trends, predictors, and impact of systemic chemotherapy in small cell lung cancer patients between 1985 and 2005. Cancer 2016;122:50-60. [Crossref] [PubMed]
- Yang CF, Chan DY, Speicher PJ, et al. Role of Adjuvant Therapy in a Population-Based Cohort of Patients With Early-Stage Small-Cell Lung Cancer. J Clin Oncol 2016;34:1057-64. [Crossref] [PubMed]
- Fournel L, Falcoz PE, Alifano M, et al. Surgical management of pulmonary large cell neuroendocrine carcinomas: a 10-year experience. Eur J Cardiothorac Surg 2013;43:111-4. [Crossref] [PubMed]
- Sun JM, Ahn MJ, Ahn JS, et al. Chemotherapy for pulmonary large cell neuroendocrine carcinoma: similar to that for small cell lung cancer or non-small cell lung cancer? Lung Cancer 2012;77:365-70. [Crossref] [PubMed]
- Varlotto JM, Medford-Davis LN, Recht A, et al. Should large cell neuroendocrine lung carcinoma be classified and treated as a small cell lung cancer or with other large cell carcinomas? J Thorac Oncol 2011;6:1050-8. [Crossref] [PubMed]
- Iyoda A, Hiroshima K, Moriya Y, et al. Post-operative recurrence and the role of adjuvant chemotherapy in patients with pulmonary large-cell neuroendocrine carcinoma. J Thorac Cardiovasc Surg 2009;138:446-53. [Crossref] [PubMed]
- Rossi G, Cavazza A, Marchioni A, et al. Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFRalpha, PDGFRbeta, and MET in large-cell neuroendocrine carcinoma of the lung. J Clin Oncol 2005;23:8774-85. [Crossref] [PubMed]
- Kenmotsu H, Niho S, Ito T, et al. A pilot study of adjuvant chemotherapy with irinotecan and cisplatin for completely resected highgrade pulmonary neuroendocrine carcinoma (large cell neuroendocrine carcinoma and small cell lung cancer). Lung Cancer 2014;84:254-8. [Crossref] [PubMed]
- Eba J, Kenmotsu H, Tsuboi MLung Cancer Surgical Study Group of the Japan Clinical Oncology Group, et al. Lung Cancer Study Group of the Japan Clinical Oncology Group. A Phase III trial comparing irinotecan and cisplatin with etoposide and cisplatin in adjuvant chemotherapy for completely resected pulmonary highgrade neuroendocrine carcinoma (JCOG1205/1206). Jpn J Clin Oncol 2014;44:379-82. [Crossref] [PubMed]


