Indoor radon exposure and lung cancer risk: a meta-analysis of case-control studies
Introduction
222Rn is a naturally occurring radioactive noble gas produced by the decay of 226Ra and belonging to the decay series of 238U. Since uranium is present in the earth’s crust, radon is found everywhere in different amounts depending on geology, in rocks, soil and underground water (1,2). It is formed underground, and its fraction rapidly penetrates into the outdoor atmosphere where it is quickly diluted. On the contrary, in confined spaces such as homes and office buildings, radon can accumulate to harmful levels (3). Today, radon in buildings is considered to be the most important indoor air pollutant. Moreover, radon and its products decay are the major source of ionizing radiation of natural origin for general population and it is considered a risk factor for lung cancer if inhaled in high concentration for a long period (4-6). After inhalation, radon is almost completely exhaled due to its long half-life (3.82 d) it is an inert gas while its progenies, in particular its daughters with short half-life 218Po and 214Po, are electrically charged so they can be attached to dust or smoke particles in indoor air. Once inhaled these particles migrate to lungs where, decaying, they irradiate the tissue damaging cells increasing the probability to get a lung cancer (7,8). The fraction of lung cancer attributable to radon indoor is estimated to be between 3% and 14% depending on the average radon concentration in the concerned country and the calculation methods (8). To better understand the mechanisms of the effects of ionizing radiation on humans, the World Health Organization recommend study in vitro on lymphocytes to estimate the frequency and spectrum of chromosomal aberrations as of the effects of exposure to ionizing radiation (9). Druzhinin et al. (10) conducted a study to assess the effect of exposure to radon by evaluating the frequency and type of chromosomal aberrations differences between groups of not exposed and exposed children to a radon concentration of 468±77 Bq/m3 during the years of investigations. The results show a significant increase of the frequencies of single and double fragmentations, chromosome interchanges, number of aberrations chromatid and chromosome type in the exposed group. Worldwide, many epidemiological studies have been carried out to estimate the association between radon exposure in houses and lung cancer for general population. However, the association between residential radon and lung cancer risk is still inconclusive. To summarize the results three meta-analyses were performed by Lubin and Boice (11), Pavia et al. (12) and Zhang et al. (13). All three meta-analyses showed a significant positive association between radon indoor and lung cancer risk. However, as regards these meta-analyses, more recent case-control studies of radon and lung cancer were published offering new information and more conclusions. In this paper authors reported the results of meta-analyses of 25 case-control studies conducted all around the world in the last 15 years. The decision to select only case-control studies was determined by the fact that many studies of this type have been conducted in many parts of the world to evaluate the association between the residential radon exposure and lung cancer risk. Moreover, these studies have provided information on the synergistic effect of the exposure to residential radon and other risk factors, such as smoking, and the risk of developing lung cancer.
Materials and methods
Study selection for the meta-analysis
The systematic literature search was carried out in PubMed, Web of Science, and Google Scholar to identify relevant studies published in English until January 2016. The key words used for the search were: “radon”, “lung cancer”, “radon epidemiology” and “radon case-control studies”. Moreover we supplemented this search checking the reference lists of the identified manuscripts to verify if the database search was incomplete.
Inclusion criteria
The most relevant studies were selected for the meta-analysis on the basis of the following inclusion criteria: (I) full-text published article; (II) case-control study with a hospital-based or population-based design; (III) examined residential exposure to radon with passive alpha-track detectors by means of measurements of at least one month; (IV) lung cancer cases histologically confirmed; (V) relative risks (RR) with their corresponding 95% confidence intervals (CIs) reported; (VI) all authors independently selected eligible studies.
Data extraction
Data extracted from selected studies were: (I) the first author’s name; (II) year of publication; (III) country where studies were carried out; (IV) study period; (V) sample size (controls and cases); (VI) sex, age range and smoking habits; (VII) radon dosimetry including detector type, duration of measurements, place of measurements; (VIII) RR with corresponding 95% CI; (IX) absolute latitude of the study location.
Statistical analysis
Meta-analysis was performed using the metafor package (14) of the R Statistical Package (The R Project for Statistical Computing: https://www.r-project.org/). The package includes functions for fitting the meta-analytic fixed- and random-effects models and allows for the inclusion of moderators variables (study-level covariates). Dose-response association of residential radon exposure with lung cancer risk with 95% CI was calculated by the method of trend estimation from summarized dose-response data (15-18). To obtain a pooled functional relation, the study-specific trends were combined according to principles of multivariate random-effects meta-analysis. Covariances of log RRs were used to efficiently estimate an exposure-disease relation by a collection of functions of the dosresmeta R package (19). Data from every single study in a dose-response meta-analysis were reconstructed by using the Greenland and Longnecker method obtaining Cases-Controls Ratios. Statistical heterogeneity between studies was assessed with the Q test, I2 statistic (total heterogeneity/total variability), and H2 statistic (total variability/sampling variability). Heterogeneity was considered significant when the P value was <0.05. A random-effects model was fitted to the data used. When statistical heterogeneity was observed, to control for the influence of potential moderators a meta-analytic mixed-effects model was used together with the sample size, case-control ratio, study design, duration of exposure, sex, follow-up time and the absolute latitude of the study location as potential predictors. Restricted maximum-likelihood estimation was used when estimating τ2, the (total) amount of residual heterogeneity among the true effects. The average true effect and the coefficients βji of the j-th moderator variable for the i-th study were estimated via weighted least squares with weights equal to , where vi denotes the sampling variance and denotes the estimate of τ2. Publication bias was evaluated with the contour-enhanced funnel plot and funnel plot asymmetry with the regression test (the Egger’s test). Leave-one-out sensitivity analysis was carried out by sequentially omitting individual studies to explore whether the results were significantly influenced by a specific study.
Results
By searching in all WEB databases about 318 studies were found. After a first screening many studies were excluded because they were studies on miners, not control-case studies and because they did not have enough information about radon exposure. Finally, 25 case-control studies on potential association between residential radon exposure and lung cancer published between 1990 and 2014 were considered eligible and used for meta-analysis (20-44). In Table 1 the characteristics of published studies on radon exposure and lung cancer risk included in the meta-analysis are reported. Of these studies 18 were population-based case-controls (20,21,23-38), 6 were hospital-based case-controls (39-44) and one both of them (22). Fourteen studies were conducted in Europe (22,26-28,32-35,37,40,41,43,44), eight in North America (21,23,24,29,30,39,40,42) and three in Asian region (20,31,36). Overall 13,569 cases and 22,701 controls were enrolled. All case-control studies involved in the three previous meta-analyses were included in this study (20-42), only newer papers (43,44) were added.
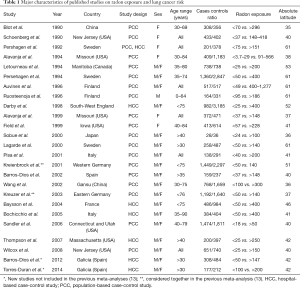
Full table
Risk estimation—random-effects model
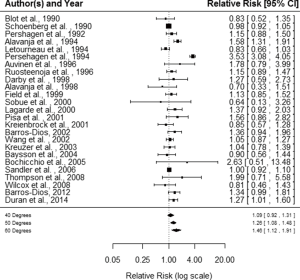
Fitting a random-effects model to the data the estimated average log RR is equal to =0.1773 (95% CI, 0.0236–0.3310). For easier interpretation, it may be useful to transform these values back to the RR scale through exponentiation, RR = exp () =1.19 with 95% CI, 1.02–1.39. A graphical overview of the results can be obtained by creating a forest plot shown in Figure 1. The null hypothesis H0: µ =0 can be clearly rejected (z =2.261, P=0.0238).
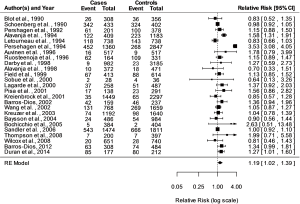
Model without moderators
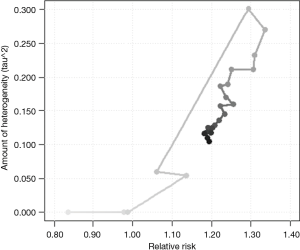
The I2 statistic, 88.86%, estimates how much of the total variability in the effect size estimates (which is composed of heterogeneity and sampling variability) can be attributed to heterogeneity among the true effects. The H2 statistic, 8.98, is the ratio of the total amount of variability in the observed outcomes to the amount of sampling variability. The test for heterogeneity (Q =325.331, df =24, P<0.0001) suggests considerable heterogeneity among the true effects. Figure 2 illustrates the results from a cumulative meta-analysis, i.e., the accumulation of evidence (RR) of lung cancer risk, plotting the estimate of the average effect against the estimated amount of heterogeneity as each study is added in chronological order to the analysis.
Detecting bias in meta-analysis
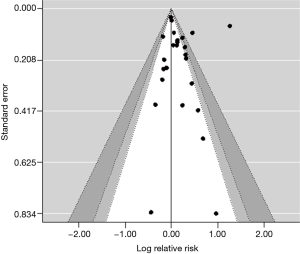
Publication bias was valuated from the contour-enhanced funnel plot (Figure 3). The Funnel plot shown in Figure 3 is more useful for detecting publication bias due to the suppression of non-significant findings. The Egger’s test was used for funnel plot asymmetry. It is a weighted regression model with multiplicative dispersion and standard error as predictor, giving: t =0.4393, df =23, P=0.6645, not suggesting asymmetry in the funnel plot.
Influential case diagnostics
Using the trim and fill method, a nonparametric (rank-based) data augmentation technique, it was possible to estimate the number of studies missing from a meta-analysis due to the suppression of the most extreme results on one side of the funnel plot. The results for a random-effects model indicate one missing study on the right side. The test for heterogeneity (Q =325.9484, df =25, P<0.0001) shows that the effect is statistically significant.
The exclusion of one study at a time, to test if it leads to considerable changes in the fitted model, showed that the pooled estimate of indoor radon exposure and lung cancer risk did not vary substantially. With the exclusion of each study, P values for the test statistics were always <0.05.
Model with moderators—mixed-effects model
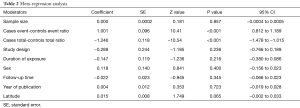
A meta-regression analysis was conducted to test if part of the heterogeneity might be due to the influence of moderators. Results are shown in Table 2. As a matter of fact, sample size, study design, duration of exposure, sex, follow-up time, year of publication, and the absolute latitude of the study location (but see below for more explanation) were not major contributors to the observed heterogeneity. Cases Event-Controls Event Ratio, and Cases Total-Controls Total Ratio (with reference to numbers reported in Figure 1) were instead the major contributors to the observed heterogeneity. The test for residual heterogeneity is now not significant (QE =3.276, df =17, P=0.99), with τ2, the estimated amount of residual heterogeneity, ≈0 (SE =0.0063), indicating no other moderator is influencing the radon indoor effectiveness to lung cancer risk. The test of moderators is obviously significant (QM =322.05, df =7, P<0.0001). Both moderators accounted for total amount of heterogeneity, R2 ≈100. Approximately half of this value was obtained if just one of the two moderators was considered in meta-regression analysis. Noteworthy, the two moderators, Cases Event-Controls Event Ratio and Cases Total-Controls Total Ratio, are poorly correlated (ρ=0.37), so avoiding multicollinearity effects in meta-regression.
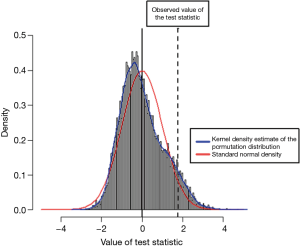
Full table
Study location and RR
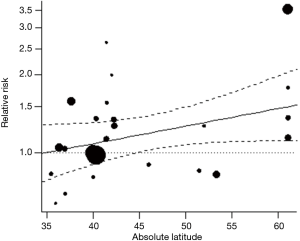
Forest plot in Figure 4 shows the estimated average RR at various degrees absolute latitude. Study location analysis showed that radon exposure was associated with increased risk for lung cancer from 40 degrees absolute latitude (RR, 1.09; 95% CI, 0.92–1.31), to 50 degrees (RR 1.26; 95% CI 1.08–1.48), to 60 degrees (RR, 1.46; 95% CI, 1.12–1.91). Figure 5 shows the RRs of the individual studies plotted against a quantitative predictor, the absolute latitude. The predicted average RR based on a mixed-effects model is added to the plot, with corresponding 95% CI bounds. Figure 6 shows a histogram of the permutation distribution of the test statistic for absolute latitude, together with the standard normal density (in red) and a kernel density estimate of the permutation distribution (in blue). The Figure shows that the tail area under the permutation distribution is larger than under the standard normal density (hence, the larger P value in this case). There is a difference of 0.011 between the two permutation P values, such that P becomes =0.054, making Latitude a significant contributor to the observed heterogeneity, unlike the value appearing in Table 2.
Discussion
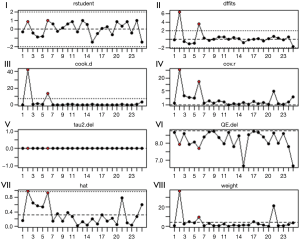
Figure 7 shows various diagnostic measures when each study is removed in turn, which is a plot of the (I) externally standardized residuals, i.e., residuals divided by their estimated standard errors (like t-statistics). Observations with values larger than 3 in absolute value are considered outliers; (II) DFFITS values, that are a measure of how much an observation has effected its fitted value from the regression model. Values larger than 2*sqrt [(k+1)/n] in absolute value are considered highly influential; (III) Cook’s distances, measuring aggregate impact of each observation on the group of regression coefficients, as well as the group of fitted values. Values larger than 4/n are considered highly influential; (IV) covariance ratios, that are a measure of the impact of each observation on the variances (and standard errors) of the regression coefficients and their covariances. Values outside the interval 1±3(k+1)/n are considered highly influential; (V) leave-one-out estimates of the amount of heterogeneity; (VI) leave-one-out values of the test statistics for heterogeneity; (VII) hat values, that are a measure of how far an observation is from the others in terms of the levels of the independent variables (not the dependent variable). Observations with values larger than 2(k+1)/n are considered to be potentially highly influential, where k is the number of predictors and n is the sample size; (VIII) weights (in %) given to the observed outcomes during the model fitting. Some of them may suggest that studies 4 and 6 (in red) (RR, 1.58; 95% CI, 1.31–1.91) and (RR, 3.53; 95% CI, 3.08–4.05) respectively, may be considered outliers. However, instead of just removing those studies, one should examine them in detail to determine what the reason may be for their unusual results. They have considerable influence on the fit of the model (the plot of the Cook’s distances and DFFITS values show this most clearly). Study 4 has in particular high leave-one-out values of the test statistics for heterogeneity and study 6 of hat values. On the other hand, removing study 4 would yield large change in the amount of covariance ratios values, meanwhile both studies do not show unusual influence on the model weights. According to (45-47), outliers and influential cases can actually reveal patterns that may lead to new insights about study characteristics. For these reasons, taking account of the reported leave-one-out sensitivity analyses, and considering the statistical significance of study 6, we decided not to remove studies 4 and 6. Moreover, the present work shows that the RR of lung cancer may depend on the absolute latitude of the residential exposure to radon. New coming studies may bear out such trend. A clue in this direction is the fact that the tail area under the permutation distribution is larger than under the standard normal density. As a matter of fact, indoor doses depend primarily on radio-active content of construction materials and on the attenuation of outside radiation by roofs and walls. The correlation of latitude with radon may be due also to other determinants of lung cancer risk: although levels at equatorial latitudes should reflect higher ventilation rates because of higher average indoor temperatures, the general scatter in the results of concentrations of radon indoors in various countries in which measurements have been made in relation to latitude, indicated that many other factors are involved. Lagarde and Pershagen reported an increase of the county-mean radon levels (Bqm−3) against latitude (48).
Conclusions
Present meta-analysis revealed that indoor exposure to radon may be associated with an effective risk of lung cancer which variates with absolute latitude. Nevertheless, further studies are needed to obtain a definitive conclusion and to determine the mechanisms underlying this association. It is far from clear, however, if the increased cancer risks reported in the literature also for other sites than the lung can be attributed to radon and progeny or concomitant gamma radiation.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Durante, Giusi I. Forte, Giorgio Russo) for the series “Radiobiological models towards a personalized radiation oncology” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.05.42). The series “Radiobiological models towards a personalized radiation oncology” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Trută-Popa LA, Cosma C, Hofmann W. Lung cancer attributed to radon exposure, for the general population: Estimation and prevention. Risk Assessment and Management. Wyoming, USA: Editura Academy Publish, 2012;422-45.
- Obed RI, Ademola AK, Ogundare FO. Radon measurements by nuclear track detectors in dwellings in Oke-Ogun area, South-Western, Nigeria. Radiat Prot Dosimetry 2012;148:475-81. [Crossref] [PubMed]
- Sarra A, Nissi E, Palermi S. Residential radon concentration in the Abruzzo region (Italy): a different perspective for identifying radon prone areas. Environ Ecol Stat 2012;19:219-47.
- . Protection against radon-222 at home and at work. A report of a task group of the International Commission on Radiological Protection. Ann ICRP 1993;23:1-45. [PubMed]
- United Nation Scientific Committee on the Effects of Atomic Radiation Report. Sources and effects of ionizing radiation. Annex B: Exposure due to Natural Radiation Sources, Vol. 1 United Nation 2000.
- Sánchez AM, Pérez Jde L, Sánchez AB, et al. Radon in workplaces in Extremadura (Spain). J Environ Radioact 2012;107:86-91. [Crossref] [PubMed]
- National Research Council (US) Committee on Health Risks of Exposure to Radon (BEIR VI). 1999, Health Effects of Exposure to Radon. Washington, DC: National Academy Press. Available online: https://www.nap.edu/read/5499/chapter/1
- WHO Hand Book on Indoor Radon: A Public Health Perspective. 2009. Available online: http://apps.who.int/iris/bitstream/10665/44149/1/9789241547673_eng.pdf
- WHO, Biomarkers and risk assessment: concepts and principles. IPCS Environmental Health Criteria, 1993. Available online: http://apps.who.int/iris/bitstream/10665/39037/1/9241571551-eng.pdf
- Druzhinin VG, Sinitsky MY, Larionov AV, et al. Assessing the level of chromosome aberrations in peripheral blood lymphocytes in long-term resident children under conditions of high exposure to radon and its decay products. Mutagenesis 2015;30:677-83. [Crossref] [PubMed]
- Lubin JH, Boice JD Jr. Lung cancer risk from residential radon: meta-analysis of eight epidemiologic studies. J Natl Cancer Inst 1997;89:49-57. [Crossref] [PubMed]
- Pavia M, Bianco A, Pileggi C, et al. Meta-analysis of residential exposure to radon gas and lung cancer. Bull World Health Organ 2003;81:732-8. [PubMed]
- Zhang ZL, Sun J, Dong JY, et al. Residential radon and lung cancer risk: an updated meta- analysis of case-control studies. Asian Pac J Cancer Prev 2012;13:2459-65. [Crossref] [PubMed]
- Viechtbauer W. metafor: Meta-Analysis Package for R. Available online: https://cran.r-project.org/web/packages/metafor/index.html
- Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301-9. [Crossref] [PubMed]
- Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata Journal 2006;6:40-57.
- Hamling J, Lee P, Weitkunat R, et al. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 2008;27:954-70. [Crossref] [PubMed]
- Orsini N, Li R, Wolk A, et al. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 2012;175:66-73. [Crossref] [PubMed]
- Crippa A, Orsini N. Dose-response meta-analysis of differences in means. BMC Med Res Methodol 2016;16:91. [Crossref] [PubMed]
- Blot WJ, Xu ZY, Boice JD Jr, et al. Indoor radon and lung cancer in China. J Natl Cancer Inst 1990;82:1025-30. [Crossref] [PubMed]
- Schoenberg JB, Klotz JB, Wilcox HB, et al. Case-control study of residential radon and lung cancer among New Jersey women. Cancer Res 1990;50:6520-4. [PubMed]
- Pershagen G, Liang ZH, Hrubec Z, et al. Residential radon exposure and lung cancer in Swedish women. Health Phys 1992;63:179-86. [Crossref] [PubMed]
- Alavanja MC, Brownson RC, Lubin JH, et al. Residential radon exposure and lung cancer among nonsmoking women. J Natl Cancer Inst 1994;86:1829-37. [Crossref] [PubMed]
- Létourneau EG, Krewski D, Choi NW, et al. Case-control study of residential radon and lung cancer in Winnipeg, Manitoba, Canada. Am J Epidemiol 1994;140:310-22. [Crossref] [PubMed]
- Pershagen G, Akerblom G, Axelson O, et al. Residential radon exposure and lung cancer in Sweden. N Engl J Med 1994;330:159-64. [Crossref] [PubMed]
- Auvinen A, Mäkeläinen I, Hakama M, et al. Indoor radon exposure and risk of lung cancer: a nested case-control study in Finland. J Natl Cancer Inst 1996;88:966-72. [Crossref] [PubMed]
- Ruosteenoja E, Mäkeläinen I, Rytömaa T, et al. Radon and lung cancer in Finland. Health Phys 1996;71:185-9. [Crossref] [PubMed]
- Darby S, Whitley E, Silcocks P, et al. Risk of lung cancer associated with residential radon exposure in south-west England: a case-control study. Br J Cancer 1998;78:394-408. [Crossref] [PubMed]
- Alavanja MC, Lubin JH, Mahaffey JA, et al. Residential radon exposure and risk of lung cancer in Missouri. Am J Public Health 1999;89:1042-8. [Crossref] [PubMed]
- Field RW, Steck DJ, Smith BJ, et al. Residential radon gas exposure and lung cancer: the Iowa Radon Lung Cancer Study. Am J Epidemiol 2000;151:1091-102. [Crossref] [PubMed]
- Sobue T, Lee VS, Ye W, et al. Residential randon exposure and lung cancer risk in Misasa, Japan: a case-control study. J Radiat Res 2000;41:81-92. [Crossref] [PubMed]
- Lagarde F, Axelsson G, Damber L, et al. Residential radon and lung cancer among never-smokers in Sweden. Epidemiology 2001;12:396-404. [Crossref] [PubMed]
- Pisa FE, Barbone F, Betta A, et al. Residential radon and risk of lung cancer in an Italian alpine area. Arch Environ Health 2001;56:208-15. [Crossref] [PubMed]
- Kreienbrock L, Kreuzer M, Gerken M, et al. Case-control study on lung cancer and residential radon in western Germany. Am J Epidemiol 2001;153:42-52. [Crossref] [PubMed]
- Barros-Dios JM, Barreiro MA, Ruano-Ravina A, et al. Exposure to residential radon and lung cancer in Spain: a population-based case-control study. Am J Epidemiol 2002;156:548-55. [Crossref] [PubMed]
- Wang Z, Lubin JH, Wang L, et al. Residential radon and lung cancer risk in a high-exposure area of Gansu Province, China. Am J Epidemiol 2002;155:554-64. [Crossref] [PubMed]
- Kreuzer M, Heinrich J, Wölke G, et al. Residential radon and risk of lung cancer in Eastern Germany. Epidemiology 2003;14:559-68. [Crossref] [PubMed]
- Baysson H, Tirmarche M, Tymen G, et al. Indoor radon and lung cancer in France. Epidemiology 2004;15:709-16. [Crossref] [PubMed]
- Sandler DP, Weinberg CR, Shore DL, et al. Indoor radon and lung cancer risk in connecticut and utah. J Toxicol Environ Health A 2006;69:633-54. [Crossref] [PubMed]
- Wilcox HB, Al-Zoughool M, Garner MJ, et al. Case-control study of radon and lung cancer in New Jersey. Radiat Prot Dosimetry 2008;128:169-79. [Crossref] [PubMed]
- Bochicchio F, Forastiere F, Farchi S, et al. Residential radon exposure, diet and lung cancer: a case-control study in a Mediterranean region. Int J Cancer 2005;114:983-91. [Crossref] [PubMed]
- Thompson RE, Nelson DF, Popkin JH, et al. Case-control study of lung cancer risk from residential radon exposure in Worcester county, Massachusetts. Health Phys 2008;94:228-41. [Crossref] [PubMed]
- Barros-Dios JM, Ruano-Ravina A, Pérez-Ríos M, et al. Residential radon exposure, histologic types, and lung cancer risk. A case-control study in Galicia, Spain. Cancer Epidemiol Biomarkers Prev 2012;21:951-8. [Crossref] [PubMed]
- Torres-Durán M, Ruano-Ravina A, Parente-Lamelas I, et al. Lung cancer in never-smokers: a case-control study in a radon-prone area (Galicia, Spain). Eur Respir J 2014;44:994-1001. [Crossref] [PubMed]
- Light RJ, Pillemer DB. Summing Up: The Science of Reviewing Research. Cambridge, MA: Harvard University Press;1984.
- Viechtbauer W. Confidence intervals for the amount of heterogeneity in meta-analysis. Stat Med 2007;26:37-52. [Crossref] [PubMed]
- Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Res Synth Methods 2010;1:112-25. [Crossref] [PubMed]
- Lagarde F, Pershagen G. Parallel analyses of individual and ecologic data on residential radon, cofactors, and lung cancer in Sweden. Am J Epidemiol 1999;149:268-74. [Crossref] [PubMed]