The role of the tumor microenvironment in bladder cancer development and progression
Introduction
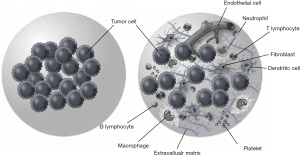
Urothelial cell carcinoma (UCC) of the urinary bladder, the most common form of bladder cancer (BC), is one of the leading causes of cancer-related death worldwide, with an estimated 429,800 new cases and 165,100 deaths in 2012 (1). BC is an intricate malignancy with a variable natural history and clinical behavior. Noninvasive, well-differentiated tumors are relatively indolent, but T1 high-grade BC and muscle invasive BC (MIBC) are life-threatening (2). Although a multidisciplinary approach has been developed, treatment and management remains challenging and controversial (3). If we are to improve clinical outcome, the mechanisms underlying tumor invasion, metastasis, and treatment resistance need to be elucidated (4). Many pathological changes in solid tumors are caused by accumulation of genetic mutations and epigenetic molecular alterations. The last few decades of cancer research have focused on identifying oncogenes and tumor suppressors that have tumorigenic roles (5). Until recently, the effects of the surrounding stromal tissue have been largely ignored. However, tumor progression is profoundly influenced by the environment surrounding transformed cells (6). It is now clear that cancer is not only a disease of uncontrolled cell growth, but also of aberrant tissue development (7). The interplay between tumor cells and their microenvironment is one of the key determinants of cancer development and progression and metastasis (8,9). As shown in Figure 1, The tumor microenvironment (TME) is a dynamic network that includes cancer cells, stromal tissue (immune cells, fibroblasts, myofibroblasts, cytokines, and vascular tissue), and the surrounding extracellular matrix (ECM) (10). A tumor alters the mechanical properties of the microenvironment to create favorable conditions for proliferation, and the microenvironment can determine tumoral cell morphology, function, aggressiveness, and response to treatment, as well as provide an accurate assessment of a patient’s prognosis (11,12). The concepts of tumor-stromal interactions and a microenvironmental niche have profound consequences with respect to tumor growth and metastasis; therefore, understanding these factors will allow us to develop new therapeutic strategies that will provide ground-breaking improvements in the treatment of UCC of the bladder (13).
This review discusses recent research into tumor-stromal crosstalk during pathogenesis of UCC of bladder, along with the roles played by the ECM, angiogenesis, endothelial cells, and cellular or and soluble components of the TME during development and progression of UCC. In addition, we provide a brief overview of TME-targeted anticancer strategies such as chemotherapy, chemo-immunotherapy, and immunotherapy.
Basic concepts about the role of the TME in cancer
Cancer tissue is a complex entity that comprises tumor cells and the surrounding stroma, which is populated by different types of mesenchymal cells, and the ECM (10). Cancer has been long viewed as a disease consisting of transformed cells that acquire autonomous hyperproliferative, invasive, and immortal phenotypes (9). Accordingly, therapeutic anticancer strategies have focused on targeting the tumor cell itself (6). Emerging evidence indicates that, to control cancer effectively, we need to consider tumorigenesis and tumor progression not as a cell autonomous, cancer cell-centered condition, but rather as a disease involving complex heterotypic multicellular interactions within a newly formed tissue, i.e., cancerous tissue (6,9). To better understand the complex interplay between cells and non-cellular stroma in UCC, we will summarize the components of the TME.
The stroma comprises many different cell types (fibroblasts/myofibroblasts, glial, epithelial, fat, vascular, smooth muscle, and immune cells), along with the ECM and extracellular molecules (14). While none of these cells are malignant in themselves, components within the immediate environment and their interactions with each other and with cancer cells (either directly or indirectly) result in acquisition of an abnormal phenotype and altered functions (14). This abnormal interplay comprising cell-cell contact and active molecular crosstalk further drives the cancer stroma phenotype, resulting in permanent alterations in cellular function (14,15). Production of growth factors and chemokines by fibroblasts and immune cells is altered, leading to direct stimulation of tumor cell growth and recruitment of precursor cells, which themselves show abnormal growth and proliferation (16). Malformed tumor vessels contribute to tumor hypoxia, acidosis, and increased interstitial fluid pressure (14,17). The tumor in turn responds by expressing a unique repertoire of genes, which in turn leads to cell growth, invasion, and (ultimately) metastasis. The unique interplay between the tumor and the TME has been the target of recent molecular strategies.
The interplay between the cells within a solid tumor and surrounding non-neoplastic cells stimulates the vital processes angiogenesis, invasion, and immune surveillance. Endothelial cells are a major cellular component of tumor vessels (18). The interaction between cancer cells and endothelial cells plays an essential role in cancer cell intravasation and migration across endothelial barriers (19). Fibroblasts and macrophages are the largest populations of non-neoplastic cells and inflammatory cells, respectively. In addition, the microenvironment determines the drug sensitivity of tumor cells. Macrophages, fibroblasts, and endothelial cells are vital components of the TME and may increase drug resistance of tumors (13).
Another evolving paradigm in BC biology is the concept of the cancer stem cell (CSC) and epithelial-mesenchymal transition (EMT). CSCs are capable of indefinite self-renewal and diverse differentiation, leading to the production of all cell types and thereby the generation of tumor heterogeneity. CSCs are thought to driver of key processes in tumor initiation, progression, as well as in the refractory to anticancer drug (20). Acquisition of stemness involves EMT, in which epithelial cells are transformed into a mesenchymal phenotype characterized by increased capacities for migration, invasiveness, and resistance to apoptosis (21). An increasing number of studies have reported that TME may involve in the activation of EMT in tumor cells, as well as mutually interact with CSCs (20,22). Taken together, the interactions between CSC/ EMT programs and the microenvironment offers an opportunity to investigate the nature of intra-tumoral heterogeneity and a possible mechanistic basis for anticancer drug resistance.
The ECM in UCC of the bladder
The bladder mucosa is lined by the urothelium, which is a stratified, transitional type of epithelium three to four cells deep and comprising cuboidal or columnar basal cells, intermediate cells, and superficial squamous cells (23). The most superficial layer of the epithelium is the only fully differentiated layer and as such forms an impermeable barrier between the lumen and the bloodstream to prevent reabsorption of harmful waste products or pathogens. The intermediate cell layer is highly proliferative, and therefore enables rapid cell regeneration in response to injury or infection of the organ or tube in which it resides. These cells contain a prominent Golgi apparatus and an array of membrane-bound vesicles (24), whose function is to package and transport proteins such as keratin to the superficial cell layer. The basal layer contains cuboidal cells that allow constant renewal of the epithelium. All epithelial cells have numerous microvilli and contain vesicles characteristic of transitional epithelium (bundles of cytoplasmic filaments, microtubules, and numerous free ribosomes) (23).
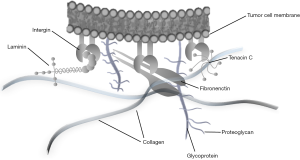
The ECM is a network of macromolecules that surrounds cells and is a substantial component of the cellular microenvironment (25). The ECM comprises structural proteins such as collagens, elastin, and laminins; glycoproteins such as fibronectin, vitronectin, and tenascin; a variety of other proteins such as proteolytic enzymes [e.g., matrix metalloproteinases (MMPs)] and their inhibitors; and proteoglycans. Tumor cells attach to specific glycoproteins within the ECM (e.g., fibronectin, collagen, and laminin) via integrins or other cell surface receptors (Figure 2) (26). Three-dimensional culture models developed to investigate interactions between the ECM and BC cells show that the ECM plays a crucial role in modulating the phenotype of BC cells (27). During progression to invasive cancer, complex interactions are required if a cell is to cross the basal membrane and invade the surrounding tissue and vessel walls. Many studies suggest that the capacity of tumor cells to interact with the ECM is a crucial component of the metastatic process (25).
Collagens play an important role as a scaffold that maintains tissue structure (26). Collagens are either organized as fibrils in tissues exposed to shear, tensile, or pressure forces (i.e., tendons, bone, cartilage, and skin) or form networks (e.g., collagen IV, which is an important component of the basal membrane) (28). Collagens are usually synthesized by mesenchymal cells such as fibroblasts and myofibroblasts, although collagen IV is also produced by adjacent epithelial cells (29). In hollow organs, the most important interstitial collagens are collagens III and I. In the normal bladder wall, these two types are mainly expressed in the lamina propria and around smooth muscle bundles and nerves (28). Interstitial collagens are thought to be involved in infiltration by individual rat BC cell lines (28,30). Mori and colleagues suggest that inactivation of the collagen type I α2 (COL1A2) gene (which encode type I collagen) via CpG hypermethylation, may contribute to proliferation and migration of BC cells (31). Brooks et al. examined the association between expression of COL1A1 and COL1A2 mRNA and cancer progression in a multi-center cohort of 189 patients with non-muscle invasive BC (NMIBC). High expression of COL1A1 and COL1A2 mRNA is associated significantly with poor progression-free and overall survival (32). Increased expression of type I collagen protein near the tumor-ECM boundary showed a significant association with NMIBC progression. The authors suggested that alterations in the ECM microenvironment, particularly type I collagen, likely contribute to BC progression (32). Type IV collagen forms a network that forms the architectural skeleton of basement membranes, and laminin plays an important role in anchoring epithelial cells to type IV collagen (33). Loss of collagen IV expression from the basal membrane is associated with worse overall survival and a tendency towards progression. Daher et al. investigated the collagen IV staining pattern in a group of invasive cancers and found that widely absent or fragmented staining in more than 5% of the tumor area was predictive of worse survival (34). Collagen VII has been studied in UCC, together with expression of integrin α6β4. In the normal bladder, the α6β4 integrin co-localizes with collagen VII at the junction of the basolateral surface of the basal urothelial cells and the lamina propria. Liebert et al. observed deranged co-localization of the hemidesmosomal anchoring complex in almost all BC samples, and found that the degree of derangement is greater in invasive cancers (35). However, alteration of collagen stainability may be of little practical importance in terms of the diagnosis and prognosis of UCC, since it is particularly sensitive to inflammatory conditions (28).
Laminins are the major intrinsic component of the basement membrane and are involved in cellular adhesion to the basement membrane and ECM. The laminin molecule is built of three disulfide-linked chains (five α, three β, and three γ isoforms) that form a characteristic cross shape (36). The effects of laminins are mediated through binding to integrins, and their most important function seems to be the interaction between epithelial cells and the ECM. Aberrant synthesis, chain composition, and proteolytic modifications are important for the interaction between malignant cells and the ECM (28). In BC, the distribution of laminins has been studied to assess infiltrative behavior, detect early invasion, and evaluate the presence of tumor-derived basal membranes. Basement membranes are thought to form a protective barrier against initial infiltration of tissues by malignant cells. Laminin-5 comprises three subunits, α3, β3, and γ2, the latter two being unique to this isoform. The γ2 chain is a specific target of MMP-2, and its cleavage is critical to cell migration during tumor invasion and tissue remodeling (37). However, in carcinomas, a dynamic interaction occurs at the interface between tumor cells and the surrounding mesenchymal stroma, and the basement membrane is not a static structure; indeed, the latter is characterized by constant deposition and degradation of its components (38).
Fibronectin and tenascin C is glycoprotein components of the ECM that seem to have competitive functions; some have suggested that this competitive relationship is important for cellular function (25). Tissue fibronectin is found in connective tissue in close apposition to the basement membrane. Few studies have examined fibronectin expression in BC tissue, urine, and blood samples, or in tissue homogenates. Ioachim et al. measured the immunohistochemical expression of the ECM components tenascin, fibronectin, collagen type IV, and laminin in UCC, and found that stromal tenascin expression showed a positive correlation with proliferative activity, and with expression of fibronectin and collagen type IV, suggesting that fibronectin is associated with proliferation, invasion, and angiogenesis (25). They also observed a longer tumor-free interval for patients with low levels of tenascin than for those with high levels of tenascin (25). Katayama et al. reported that gastrointestinal cancer patients have higher levels of urinary fibronectin than healthy controls (39). An automatic assay, called bladder tumor fibronectin, was developed but was limited by lack of sensitivity for low-grade tumors and a lack of specificity with respect to benign conditions (28). Tenascin C is an ECM glycoprotein expressed transiently during embryogenesis. Expression of tenascin C is downregulated in most adult tissues, but reappears in various pathological conditions, including reparative, hyperplastic, inflammatory, and neoplastic processes (40). Tenascin C comprises six disulfide-linked subunits. Each subunit consists of four repeated structural domains that include epithelial growth factor-like repeats, an N-terminal domain, fibronectin III homology repeats, and C-terminal globular domains shared with fibrinogen (41). Alternative splicing of tenascin C results in many different forms that contain variable numbers of additional fibronectin III repeats. Low spliced, large tenascin C is preferentially expressed during tissue remodeling processes such as embryogenesis, organogenesis, wound healing, and carcinoma development, and modulates cancer cell adhesion to the ECM (42). Functionally, tenascin C is involved in cell adhesion, migration, and growth through its interaction with fibronectin-dependent cell adhesion molecules. Tenascin C binds to the fibronectin and syndecan-4, which is necessary for cells to spread fully on fibronectin (43). By interfering with binding to fibronectin, tenascin C prevents the interaction between cells and fibronectin in synergy with integrin α5β1 (44). Brunner et al. found that diffuse tenascin C staining in the stroma of invasive tumors is associated with a significantly worse OS than negative or only moderate tenascin C expression (40). Berndt et al. evaluated differential expression of tenascin C splicing variants as a possible indicator of UCC tumor behavior. They suggested that detection of different tenascin C splicing domains could be useful for assessing muscle invasion, tumor surveillance, and target structures for antibody-based tumor detection and therapy (42). Tenascin C expression in UCC, with particular emphasis on pattern and distribution, may provide additional prognostic information, although its role in tumorigenesis and progression of BC requires further investigation.
Thrombospondins (TSP) are a family of large ECM glycoproteins comprising five members: TSP-1 to TSP-5. TSP-1 and TSP-2 have been studied extensively in terms of their antiangiogenic properties (45). Grossfeld et al. report that low TSP-1 expression is associated with high microvessel density (MVD), shorter recurrence-free survival, and worse overall survival in a study that included 163 UCC patients (46). Goddard et al. found that loss of TSP-1 was an independent prognostic factor for worse overall survival (47). In vitro studies show that TSP-1 inhibits angiogenesis induced by a BC cell line, and that TSP-1, together with tenascin C, modulates sprouting of endothelial cells (48).
Integrins are heterodimeric cell adhesion molecules that link the ECM to the cytoskeleton. The human integrin family comprises 24 members, which are the result of different combinations of 1 of 18 α- and 1 of 8 β-subunits (49). Alternative splicing of mRNA encoding α- and β-subunits, along with post-translational modification of integrin subunits, further increases the diversity of the integrin family. Combinations of chains allow the formation of a wide range of different integrin molecules (50). They mainly function as receptors for ECM proteins, including laminin, collagen VII, fibronectin, tenascin C, vitronectin, and TSP-1. The normal bladder urothelium expresses α3, αV, β1, and β4, all of which are important for impermeability of the bladder wall (28). α3-integrin is thought to be involved in modulating expression of other integrin receptors in BC. Derangement, loss, and shifts in integrin expression play an important role in invasion of malignant tumors, including UCC of the bladder (51). Derangement of α6β4 integrin in BC is associated with invasive behavior, since its loss impairs tumor cell binding to the basal membrane component collagen VII (35). Reduced expression of integrin β4 correlates with increased intraepithelial spread of tumor cells on laminin (52).
Angiogenesis and endothelial cells in urothelial carcinoma of the bladder
Angiogenesis is defined as development of new vessels from the existing vasculature. It is a normal physiological process during fetal development, the menstrual cycle, and wound healing. However, it is essential for tumor growth, invasion, and subsequent metastasis (53). This process is strictly regulated and there are several different mechanisms involved. Angiogenesis depends mainly on endothelial cell migration and proliferation. Many proangiogenic factors recruit circulating endothelial progenitors derived from bone marrow to sites of active angiogenesis (54). These create a first group of migrating cells, which further develop new capillary sprouts and finally recruit pericytes and smooth muscle cells and organize endothelial cells to ensure capillary stabilization. There is a fine balance between all the involved factors, and under normal conditions angiogenesis remains strictly controlled. During tumorigenesis, the so called “angiogenic switch” is activated and the whole process of angiogenesis becomes deregulated, resulting in increased neovascularization (55).
MVD is used to assess and identify tumor vasculature using an antibody that targets endothelial cells, and it is an independent predictor of survival in UCC patients (56). Goddard et al. determined whether MVD at the time of presentation is related to subsequent progression of NMIBC. Multivariable analysis revealed that MVD at the time of presentation was an independent prognostic indicator for subsequent disease progression (57). Ajili et al. also showed that MVD was an independent predictor of recurrence after bacillus Calmette-Guerin (BCG) immunotherapy (58). Bochner et al. analyzed MVD in 164 MIBC samples and found a high MVD to be an independent prognostic indicator for patients with MIBC (59).
Variations in oxygen tension may result in activation of different pathways, thereby generating numerous transcriptional factors. Hypoxia-inducible factor (HIF)-1 and HIF-2 are the most important transcriptional factors that regulate genes involved in responses to hypoxia (60). Under normoxic conditions, HIF interacts with the Von Hippel-Lindau protein, which is an ubiquitin ligase. HIF is degraded by the proteasome through a process called ubiquitinization. Conversely, under hypoxic conditions, HIF is not ubiquitinized and the two subunits of HIF α and β bind and activate expression of numerous genes involved in these processes (61). These genes are mainly involved in angiogenesis, and include vascular endothelial growth factor (VEGF), vascular endothelial growth factor receptor 1 (VEGFR-1), and angiopoietin-2, but other genes participate in glucose metabolism, cell adhesion and migration, proteolysis, pH regulation, and cell proliferation (62). In addition, loss of function of Von Hippel-Lindau protein leads to an absence of HIF-1α degradation, leading to constitutive activation of the above-mentioned target genes, which are ultimately responsible for angiogenesis, cell proliferation, and survival (63).
VEGF is the most important of the angiogenic stimulators. There are four main forms, each with a variety of functions such as recruitment and mitogenic stimulation of endothelial cells (64). The main ligand for tumor angiogenesis is VEGF-A, which binds to VEGFR-1 and VEGFR-2, thereby transducing major signals for angiogenesis. Other factors such as VEGF-C and VEGF-D bind to VEGFR-3, which is mainly involved in lymphangiogenesis (65). These factors act in a paracrine manner, as the tumor cells and their supporting macrophages and mesenchymal cells secrete VEGF-A, which subsequently activates its receptors on endothelial cells to promote angiogenesis. VEGF initially interacts with VEGFR-2 to increase endothelial cell proliferation, migration, and vascular permeability, followed by VEGFR-1 to assist organization of new capillary tubes (66). The important role of this factor in tumor angiogenesis is suggested by observations that tumor cells transfected with VEGF grow more rapidly and form more vascular xenografts than non-transfected controls, and that anti-VEGF antibodies inhibit tumor growth and invasion in vivo (64). The prognostic value of VEGF has been reported in a number of human cancers, including UCC of the bladder. Crew et al. noted that NMIBC with higher expression of VEGF mRNA appears to show earlier recurrence, a greater risk of stage progression, and shortened disease-free survival (67). In addition, they reported quantification of urinary VEGF in BC patients using enzyme-linked immunosorbent assays (68). Other published reports show the clinical relevance of VEGF expression in the serum of BC patients. Miyake et al. showed that elevated serum VEGF levels are an independent predictor of recurrence and disease progression in patients with NMIBC (69). Systemic therapy with Mab DC101, an inhibitor of murine VEGFR-2, inhibits the growth of human epidermoid, renal, pancreatic, and glioblastoma xenografts growing within the subcutis of athymic nude mice (70).
MMPs are a large family of proteolytic enzymes involved in breakdown of the ECM. MMPs, in particular interstitial collagenase (MMP-1), stromelysin-1 (MMP-3), stromelysin-3 (MMP-11), and the gelatinases MMP-2 and MMP-9, are found in increased amounts in tumor tissues, where they regulate tumor growth and metastasis, and promote invasion by malignant cells (71). They are activated by proenzymes, hypoxia, and acidosis, and contribute to release proangiogenic factors such as fibroblast growth factor (FGF). In addition, by degrading the basement membrane of the vascular endothelium and the ECM, they facilitate formation of new capillaries (72). In recent years, many studies have focused on the roles of MMPs in tumor invasion and metastasis. In numerous carcinomas, increased expression of MMPs is associated with a higher grade of malignancy and a poor prognosis. In BC, MMP-2 (gelatinase A, 72 kDa gelatinase) and MMP-9 (gelatinase B, 92 kDa gelatinase) are of particular importance during this step because they hydrolyze basal membrane type IV collagen (73). Margulies et al. reported elevated levels of MMP-2 in the urine of patients with BC, and Ozdemir et al. reported the same for MMP-9 (74,75). Gerhards et al. also found a significant increase in urinary MMP-2 and MMP-9 excretion in patients with BC, which was dependent on tumor stage and grade (76). Tissue inhibitors of matrix metalloproteinases (TIMPs) are natural inhibitors of MMP activity, which creates a balance between MMP/TIMP function; therefore, an increase in MMP activity and/or a decrease in TIMP function may induce MMP-dependent remodeling of the ECM and subsequent tumor invasion. TIMP-1 binds at a 1:1 ratio to inactivate MMP-2 and MMP-9, whereas TIMP-2 specifically inhibits MMP-2 activity (77). Nevertheless, this issue seems to be far more complex, as many tumors such as BC show high TIMP production, and TIMPs may have some growth-promoting effects. Hara et al. measured expression of MMP-2, MMP-9, membrane-type MMP-1, TIMP-1, and TIMP-2 mRNA in 51 NMIBC samples (78). The authors showed that elevated MMP-9 and TIMP-2 levels were independent predictors of intravesical tumor recurrence, and a paradoxical positive correlation between overexpression of TIMP-2 and a high incidence of tumor recurrence (78).
FGF is a growth and differentiation factor that plays fundamental roles in embryonic development, tissue regeneration, angiogenesis, and neoplastic transformation. It binds to heparin sulfate proteoglycans on the cell surface and ECM, becoming stabilized against proteolysis; therefore, it interacts with FGF receptors, leading to endothelial cell proliferation, regulation of integrin and cadherin expression, and modulation of cell to cell interactions (38). It also acts synergistically with VEGF to generate a significant angiogenic response in target cells (79).
Interleukin (IL)-8 is a proinflammatory cytokine initially described as a leukocyte chemoattractant but subsequently identified as a potent mitogenic, angiogenic, and growth factor. Koch et al. found that human recombinant IL-8 is a potent proangiogenic agent when implanted into a rat cornea, where it induced proliferation and chemotaxis of human umbilical vein endothelial cells. It is not clear how IL-8 exerts its angiogenic activity (80). It seems to be involved in upregulating MMP-2 expression and activity, and also acts directly on vascular endothelial cells as a survival factor. Neutralization of IL-8 using a fully humanized antibody (ABX-IL8) was effective against human melanoma cell lines as it reduced both angiogenesis and tumor growth, inhibited MMP-2 activity, and increased tumor cell apoptosis (81). The same strategy was used to treat BC cell lines and xenografts, although ABX-IL8 had no clear effect on UCC in vitro; however, it achieved a significant reduction in tumor growth in the orthotopic nude mouse model (62).
The oncoprotein, epidermal growth factor receptor (EGFR), is overexpressed in 31–48% of BC cases and is associated with a poor outcome. EGFR also plays a role in angiogenesis by regulating the activity of VEGF, IL-8, basic fibroblast growth factor (bFGF), and MMPs. Studies in BC cell lines show that EGFR inhibition reduces VEGF, IL-8, and bFGF levels, which are three of the most important proangiogenic factors (82).
Cyclooxygenase (COX) is involved in the prostaglandin synthesis pathway and exists as two isoforms: COX1 and COX2. COX2 is proinflammatory and has a proangiogenic role. COX2 increases expression of VEGF and bFGF, but also seems to stimulate antiapoptotic pathways (83).
Platelet-derived endothelial cell growth factor (PD-ECGF) was initially identified as a novel angiogenic factor present in platelets. Subsequent studies showed that PD-ECGF is identical to thymidine phosphorylase, which catalyzes the reversible breakdown of thymidine to thymine in the presence of inorganic orthophosphate (84). Sawase et al. evaluated expression of PD-ECGF in BC and observed a correlation between PD-ECGF expression and tumor grade and stage; moreover, there was a correlation with recurrence-free survival of patients with NMIBC (85).
The role of cellular and soluble components of the TME in UCC of the bladder
The TME comprises stromal cells [fibroblasts, macrophages, regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), endothelial cells, pericytes, and platelets], the ECM (inflammatory cytokines, chemokines, MMPs, integrins, and other secreted molecules) and exosomes, and establishes an autocrine-paracrine communication circuit that reinforces invasion and cancer cell metastasis via reciprocal signaling (6,9).
Cancer-associated fibroblasts (CAFs), which comprise both fibroblasts and myofibroblasts, are frequently observed in the stroma of human carcinomas, where their presence in large numbers is often associated with development of high-grade malignancies and poor prognoses (86). Under normal circumstances, fibroblasts maintain tissue structure by secreting ECM precursors. During tumorigenesis, fibroblasts transform into CAFs through crosstalk signaling between tumor cells and the surrounding stroma. CAFs perform several functions that support tumor growth, such as secreting VEGF, FGFs, PD-ECGF, and other proangiogenic signals to induce angiogenesis. CAFs can also secrete transforming growth factor β (TGF-β), which is associated with EMT, a process by which cancer cells can metastasize; EMT inhibits cytotoxic T cells and natural killer T cells. As fibroblasts, CAFs rework the ECM to include more paracrine survival signals such as insulin-like growth factor 1 (IGF-1) and IGF-2, thereby promoting survival of the surrounding cancer cells. CAFs are also associated with the Reverse Warburg Effect, in which CAFs undergo aerobic glycolysis and feed lactate to cancer cells (87). To date, the nature and role of CAFs in UCC remains poorly understood.
Myeloid-derived suppressor cells (MDSCs) are a heterogenous population of cells that consists of myeloid progenitor cells and immature myeloid cells. These cells are a mixture of immature myeloid cells, immature granulocytes, monocytes-macrophages, dendritic cells, and myeloid progenitor cells (88). Recent data from a number of groups demonstrate that these cells are responsible for tumor-mediated immune suppression in both mice and humans. MDSCs suppress T cell function via several mechanisms, including production of arginases that reduce levels of L-arginine, which is critical for normal T cell function. Reduced levels of arginine reduce T cell receptor chain expression and promote T cell dysfunction. These cells also secrete nitric oxide and reactive oxygen species, which can also suppress T cells (89).
Tumor-associated macrophages (TAMs) are the major inflammatory component of the stroma of many tumors and affect different aspects of neoplastic tissue. Macrophages are phagocytic cells that play pivotal roles in inflammation, wound healing, and tissue repair. Exposure to different molecular signals can induce two types of phenotypic differentiation: M1 (“classically activated”) and M2 (“alternatively activated”). M1 macrophages respond to cytokines such as interferon-γ, and inhibit tumor progression by expressing proinflammatory and immunostimulatory cytokines (90). However, during tumor progression in the presence of IL-4, IL-10, and IL-13, a phenotypic switch occurs and macrophages differentiate into the M2 phenotype, which secretes IL-4, IL-5, and IL-6 and increases angiogenesis, matrix remodeling, and immune suppression (91). Large numbers of studies have focused on identifying the prognostic value of TAMs in solid tumors, and most suggest that TAMs are beneficial for tumor growth and are therefore associated with a poor prognosis. The presence of TAMs correlates positively with increased vascularity and metastasis, and with decreased relapse-free and overall survival rates in breast cancer and non-small cell lung cancer (92,93). Similarly, increased infiltration by TAMS seems to be associated with a poor prognosis for BC patients. Hanada et al. investigated the prognostic value of TAMs in 40 cases of NMIBC and 23 cases of MIBC. The TAM count in MIBC was significantly higher than that in NMIBC, and there was a positive correlation between the TAM count and MVD (94). Takayama and colleagues investigated the correlation between TAMs infiltrating BC in situ and the response to intravesical BCG therapy. The authors revealed that increased infiltration of TAMs is associated with poor prognosis of bladder carcinoma in situ after intravesical BCG instillation (95).
Similar to myeloid macrophages, neutrophils contain a subpopulation of neutrophils named tumor-associated neutrophils (TANs). The literature reveals a dual role for neutrophils in tumor biology. Activated leukocytes kill tumor cells, thereby playing a beneficial, protective role in the host. By contrast, TANs promote malignancy in certain situations, e.g., by releasing growth-stimulating signals, matrix-degrading proteases, and mediators of angiogenesis. Recently, Fridlender et al. provided evidence for the existence of N1 (antitumoral) and N2 (protumoral) TANs, which are analogous to M1 and M2 macrophages, respectively (96). This neutrophil plasticity is regulated by molecules within the TME. The immunosuppressive cytokine TGF-β induces neutrophils to acquire an N2 protumoral phenotype, and the presence of TGF-β prevents generation of antitumorigenic N1 neutrophils. The antitumoral activity of N1 neutrophils includes increased expression of immuno-activating cytokines and chemokines, lower levels of arginase, a greater capacity to kill tumor cells, and activation of cytotoxic T lymphocytes (CTLs). Therefore, augmentation of N1 neutrophil numbers and activity might be therapeutically beneficial. For instance, switching off angiogenesis while increasing the local production of TGF-β may skew N2 cells towards an N1 phenotype (97).
One of the major mechanisms by which cancer cells block antitumor immune responses involves a specific class of T cells called tumor-infiltrating Tregs. In the vast majority of cases, these cells express the Forkhead box P3 (FOXP3) transcription factor. Such FOXP3+ Tregs accumulate within neoplastic lesions via several distinct mechanisms, including increased infiltration, local expansion, survival advantage, and de novo development from conventional CD4+ cells (98). Whereas Tregs in healthy peripheral organs constitute approximately 10% of total CD4+ T cells, this proportion is consistently increased in the TME, in which Tregs can account for 30–50% of CD4+ T cells. The phenotype of intratumoral Tregs appears to differ from that of circulating Tregs, and the former are also thought to promote tumor angiogenesis, thereby favoring tumor growth via immune-independent mechanisms. Various studies report that tumor infiltration by Tregs has a negative prognostic value, although this seems to be strongly influenced by other clinical and biological parameters such as tumor type, location, and stage, and presence of other immune effector cells, notably CD8+ CTLs (99).
Tumor-infiltrating lymphocytes (TILs) are host lymphocytes that appear at tumor sites; presumably, they migrate to the tumor to combat the growing malignant cells. They comprise activated T cells, natural killer cells, and non-T or non-B lymphocytes. Evidence suggests that multiple variables contribute to immune escape, including regulatory cells; inhibitory ligands on tumor cells, such as PD-L1 and B7x; soluble factors such as TGF-β and IL-10; and nutrient-catabolizing enzymes, such as indoleamine-2,3-dioxygenase (IDO) (100). Fas-ligand (FasL) is a cell surface protein [belonging to the tumor necrosis factor (TNF) family] that induces apoptosis on Fas-bearing cells when FasL binds to Fas. Fas-mediated apoptosis is dependent on activation of different members of a family of cysteinyl aspartate proteases called caspases, which includes caspase-8, caspase-9, and caspase-3, all of which are responsible for both initiation of the apoptotic cascade and mediation of cell damage. Interaction between FasL and Fas+ TILs results in cell death. Therefore, expression of FasL by tumor cells may provide an “immune privileged” mechanism by which cancer cells escape eradication by TILs. A recent study by Chopin et al. reported FasL expression in 45% of UCC samples, while no expression was observed in normal urothelium. There was a correlation between FasL expression and high tumor grade and stage (101).
Platelet numbers correlate with cancer progression and metastasis. This is largely attributable to platelet-mediated enhancement of tumor cell survival, extravasation, and angiogenesis. Platelets enable metastasis via multiple mechanisms. Tumor cells induce platelet aggregation and embolus formation, which favor survival in a stressful environment. This mechanism protects tumor cells from immune-mediated clearance and from shear stresses, which can be toxic. Platelets also form complexes with leukocytes and facilitate adhesion to endothelial cells. This adhesive cellular aggregation is able to extravasate at sites of secondary metastasis (102).
Chemokines and cytokines have pro-migratory effects upon leukocytes, endothelial cells, and epithelial cells, and as such play a fundamental role in angiogenesis, tumor cell proliferation, and metastasis in ovarian cancer. Because they are expressed by nearly all cells, cytokines stimulate cell growth, regulate cell differentiation, and modulate expression of other cytokines. The small 9–14 kDa chemokines are chemoattractive proteins. Chemokines are divided into four subfamilies (CC, CXC, C, and CX3C) based upon the number and location of conserved cysteine residues in their primary structure. To date, about 50 chemokines and 20 chemokine receptors have been identified in humans. Binding of chemokines to G protein coupled receptors activates downstream signaling cascades, thereby regulating leukocyte trafficking, adhesion to ECM molecules, and directional invasion (103). Recent evidence indicates that tumor cells express distinct, tumor type-specific, nonrandom patterns of chemokine receptors, and that signaling through these receptors is crucial for chemotactic migration, invasion, and cancer metastasis. Urinary CXCL1 levels are higher in patients with invasive BC than in those with noninvasive tumors and normal controls, a finding confirmed by analyses of secretory products from highly invasive and poorly invasive BC cell lines (104). Tests for urinary CXCL8 appear best for BC detection (better than those for MMP-9 and VEGF), showing 90% sensitivity and 86% specificity. Of the six known CXCL12 isoforms generated by alternative mRNA splicing, the beta-isoform appears to be an independent predictor of metastasis and disease-specific mortality in BC (105).
Exosomes are tiny extracellular vesicles with a diameter ranging from 30 to 100 nm. They are key mediators of intercellular communication between cells. The biogenesis of exosomes starts with an inward invagination of the plasma membrane, leading to incorporation of membrane proteins within early endosomes. The limiting membrane of the endosomes invaginates further and cytosolic proteins and RNAs are selectively targeted and enclosed within the internal vesicles to form multivesicular bodies (MVBs) within the cytoplasm. These MVBs subsequently fuse with the plasma membrane and release the exosomes outside the cell. Although exosomes are secreted by most cell types, emerging evidence suggests enhanced exosome release under pathological conditions such as tumorigenesis. Exosomes may facilitate crosstalk between tumor cells and major cell types in the TME, such as fibroblasts, endothelial cells, and immune cells, as well as non-cellular ECM components, through paracrine mechanisms (106). Welton et al. observed some differences in the protein profiles of urinary exosomes derived from patients with BC and healthy donors (107).
Clinical implications of TME in UCC of the bladder
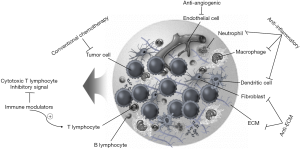
Although UCC of the bladder is a chemosensitive tumor, most BC-related deaths are caused by metastases that are resistant to conventional chemotherapy. Most patients with advanced UCC show an initial response to chemotherapy, but chemoresistant disease rapidly ensues. Therefore, new chemotherapeutic strategies must be developed if we are to improve the outcome for patients with advanced BC. Theoretically, targeting the TME is advantageous because stromal components do not develop mutations or genetic aberrations as frequently as tumor cells. As our understanding of tumor cell-stroma interactions has increased and the pathways involved have become better characterized, significant efforts have been made to identify, develop, and test therapeutic agents that interfere with the recruitment of stromal cells into the TME, with tumor cell–stromal interactions, or with specific pathways activated by the TME. Several strategies that target the TME are in clinical development: (I) antiangiogenic agents targeting VEGF, FGF, PD-ECGF, and EGFR signaling; (II) Targeting cancer-associated inflammation by inhibiting TAM and targeting the COX2, IL-6/Janus kinase (JAK)/signal transducer and activator of transcription (STAT3), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), TNF-alpha, and TGF-β signaling pathways; and (III) Immune modulators targeting the programmed cell death protein 1 (PD-1)/programmed death ligand 1 (PD-L1) pathway, cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), lymphocyte-activation gene 3 (LAG-3), T cell immunoglobulin and mucin containing protein-3 (TIM-3), B7-H3, and B7-H4/B7-Hx (Figure 3). However, despite the strong clinical rationale and availability of agents, clinical application has so far limited in UCC. Atezolizumab (Tecentriq®, Genentech), a humanized immunoglobulin monoclonal antibody specific for PD-L1, was approved by the Food and Drug Administration (FDA) in early 2016. The PD-1 inhibitor nivolumab (Opdivo®, Bristol-Myers-Squibb) is the second immune checkpoint inhibitor to be granted FDA breakthrough therapy status for advanced UCC. In June 2016, it was approved for unresectable locally advanced or metastatic UCC after progression on a platinum-based regimen. Bevacizumab is a humanized monoclonal antibody that targets VEGF and has been approved by the FDA for use in combination with chemotherapy as a standard treatment (first line and second line) for different metastatic tumors. Bevacizumab was evaluated as a first line treatment for bladder UCC in combination with GC or a dose-dense MVAC protocol (phase II trial) (108,109).
The TME is a very complex and dynamic network, so it is unlikely that one drug can ever be used to treat all tumors. Instead, it is likely that treatments for a certain type of cancer will require multiple targeting agents directed at different aspects of the tumor. The initial results are promising, and clinical trials exploring this strategy (not only for advanced disease but also in a neoadjuvant and an adjuvant setting) are currently accruing patients; future data regarding these trials are eagerly awaited and, hopefully, will help us to improve the survival and quality of life of patients with advanced or metastatic UCC.
Conclusions
Tumor-stroma interactions are mediated by complex and dynamic crosstalk between cytokines, chemokines, growth factors, enzymes, microRNAs, and other effector molecules. As our understanding of the role of the TME grows, the complexity of the interactions between cancer cells and their surrounding tissues becomes more and more evident. Signaling and effector molecules not only passively diffuse through the ECM to reach their target cells but are also transported via specialized particles such as exosomes. Even though we have made much progress in understanding the importance of the TME in cancer, several unanswered questions remain. Understanding the roles of the ECM and the microbiota, two key components of the urothelial mucosa, in the sequelae of pathogenic events that occur during development and progression of UCC will be important if we are to overcome the shortcomings of current BC treatment strategies.
Acknowledgments
Funding: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (No. NRF-2015R1A2A2A03004100).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ja Hyeon Ku, Kunyoo Shin, Minyong Kang) for the series “Bladder Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.06.48). The series “Bladder Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Enokida H, Yoshino H, Matsushita R, et al. The role of microRNAs in bladder cancer. Investig Clin Urol 2016;57:S60-76. [Crossref] [PubMed]
- Miyake M, Hori S, Morizawa Y, et al. CXCL1-Mediated Interaction of Cancer Cells with Tumor-Associated Macrophages and Cancer-Associated Fibroblasts Promotes Tumor Progression in Human Bladder Cancer. Neoplasia 2016;18:636-46. [Crossref] [PubMed]
- Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet 2009;374:239-49. [Crossref] [PubMed]
- Eccles SA, Welch DR. Metastasis: recent discoveries and novel treatment strategies. Lancet 2007;369:1742-57. [Crossref] [PubMed]
- Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell 2009;136:823-37. [Crossref] [PubMed]
- Koontongkaew S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J Cancer 2013;4:66-83. [Crossref] [PubMed]
- Strand DW, Franco OE, Basanta D, et al. Perspectives on tissue interactions in development and disease. Curr Mol Med 2010;10:95-112. [Crossref] [PubMed]
- Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 2011;17:320-9. [Crossref] [PubMed]
- Lorusso G, Rüegg C. The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem Cell Biol 2008;130:1091-103. [Crossref] [PubMed]
- Weber CE, Kuo PC. The tumor microenvironment. Surg Oncol 2012;21:172-7. [Crossref] [PubMed]
- Tadeo I, Álvaro T, Navarro S, et al. Tumor Microenvironment Heterogeneity: A Review of the Biology Masterpiece, Evaluation Systems, and Therapeutic Implications. In: Travascio F. editor. Composition and Function of the Extracellular Matrix in the Human Body. Fontana, CA: InTech; 2016.
- Fukumura D, Jain RK. Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J Cell Biochem 2007;101:937-49. [Crossref] [PubMed]
- Chen F, Zhuang X, Lin L, et al. New horizons in tumor microenvironment biology: challenges and opportunities. BMC Med 2015;13:45. [Crossref] [PubMed]
- Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem 2007;101:805-15. [Crossref] [PubMed]
- Brychtova S, Bezdekova M, Hirnak J, et al. Stromal microenvironment alterations in malignant melanoma. In: Murph M. editor. Research on Melanoma-A Glimpse into Current Directions and Future Trends. Fontana, CA: InTech; 2011.
- Goubran HA, Kotb RR, Stakiw J, et al. Regulation of tumor growth and metastasis: the role of tumor microenvironment. Cancer Growth Metastasis 2014;7:9-18. [Crossref] [PubMed]
- Watnick RS. The role of the tumor microenvironment in regulating angiogenesis. Cold Spring Harb Perspect Med 2012;2:a006676 [Crossref] [PubMed]
- Ungefroren H, Sebens S, Seidl D, et al. Interaction of tumor cells with the microenvironment. Cell Commun Signal 2011;9:18. [Crossref] [PubMed]
- Reymond N, d'Agua BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nat Rev Cancer 2013;13:858-70. [Crossref] [PubMed]
- Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 2010;29:4741-51. [Crossref] [PubMed]
- Chang JC. Cancer stem cells: Role in tumor growth, recurrence, metastasis, and treatment resistance. Medicine 2016;95:S20-5. [Crossref] [PubMed]
- Albini A, Bruno A, Gallo C, et al. Cancer stem cells and the tumor microenvironment: interplay in tumor heterogeneity. Connect Tissue Res 2015;56:414-25. [Crossref] [PubMed]
- Monis B, Zambrano D. Ultrastructure of transitional epithelium of man. Z Zellforsch Mikrosk Anat 1968;87:101-17. [Crossref] [PubMed]
- Alroy J, Merk FB, James Morré D, et al. Membrane differentiation in the Golgi apparatus of mammalian urinary bladder epithelium. Anat Rec 1982;203:429-40. [Crossref] [PubMed]
- Ioachim E, Michael M, Stavropoulos NE, et al. A clinicopathological study of the expression of extracellular matrix components in urothelial carcinoma. BJU Int 2005;95:655-9. [Crossref] [PubMed]
- Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 2012;196:395-406. [Crossref] [PubMed]
- Dozmorov MG, Kyker KD, Saban R, et al. Analysis of the interaction of extracellular matrix and phenotype of bladder cancer cells. BMC Cancer 2006;6:12. [Crossref] [PubMed]
- Brunner A, Tzankov A. The role of structural extracellular matrix proteins in urothelial bladder cancer. Biomark Insights 2007;2:418-27. [PubMed]
- Friman T. Extracellular Matrix and Connective Tissue Cells of the Tumor Microenvironment: Acta Universitatis Upsaliensis; 2010.
- Tucker GC, Boyer B, Gavrilovic J, et al. Collagen-mediated dispersion of NBT-II rat bladder carcinoma cells. Cancer Res 1990;50:129-37. [PubMed]
- Mori K, Enokida H, Kagara I, et al. CpG hypermethylation of collagen type I alpha 2 contributes to proliferation and migration activity of human bladder cancer. Int J Oncol 2009;34:1593-602. [PubMed]
- Brooks M, Mo Q, Krasnow R, et al. Positive association of collagen type I with non-muscle invasive bladder cancer progression. Oncotarget 2016;7:82609. [PubMed]
- Tanjore H, Kalluri R. The role of type IV collagen and basement membranes in cancer progression and metastasis. Am J Pathol 2006;168:715-7. [Crossref] [PubMed]
- Daher N, Abourachid H, Bove N, et al. Collagen IV staining pattern in bladder carcinomas: relationship to prognosis. Br J Cancer 1987;55:665-71. [Crossref] [PubMed]
- Liebert M, Washington R, Wedemeyer G, et al. Loss of co-localization of alpha 6 beta 4 integrin and collagen VII in bladder cancer. Am J Pathol 1994;144:787-95. [PubMed]
- Aumailley M, Gayraud B. Structure and biological activity of the extracellular matrix. J Mol Med (Berl) 1998;76:253-65. [Crossref] [PubMed]
- Ono Y, Nakanishi Y, Ino Y, et al. Clinicopathologic significance of laminin-5 γ2 chain expression in squamous cell carcinoma of the tongue. Cancer 1999;85:2315-21. [Crossref] [PubMed]
- Lu P, Takai K, Weaver VM, et al. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 2011;3:a005058 [Crossref] [PubMed]
- Katayama M, Hino F, Kamihagi K, et al. Urinary fibronectin fragments (a potential tumor marker) measured by immunoenzymometric assay with domain-specific monoclonal antibodies. Clin Chem 1991;37:466-71. [PubMed]
- Brunner A, Mayerl C, Tzankov A, et al. Prognostic significance of tenascin-C expression in superficial and invasive bladder cancer. J Clin Pathol 2004;57:927-31. [Crossref] [PubMed]
- Chothia C, Jones EY. The molecular structure of cell adhesion molecules. Annu Rev Biochem 1997;66:823-62. [Crossref] [PubMed]
- Berndt A, Anger K, Richter P, et al. Differential expression of tenascin-C splicing domains in urothelial carcinomas of the urinary bladder. J Cancer Res Clin Oncol 2006;132:537-46. [Crossref] [PubMed]
- Van Obberghen-Schilling E, Tucker RP, Saupe F, et al. Fibronectin and tenascin-C: accomplices in vascular morphogenesis during development and tumor growth. Int J Dev Biol 2011;55:511-25. [Crossref] [PubMed]
- Huang W, Chiquet-Ehrismann R, Moyano JV, et al. Interference of tenascin-C with syndecan-4 binding to fibronectin blocks cell adhesion and stimulates tumor cell proliferation. Cancer Res 2001;61:8586-94. [PubMed]
- Mongiat M, Andreuzzi E, Tarticchio G, et al. Extracellular Matrix, a Hard Player in Angiogenesis. Int J Mol Sci 2016;17:E1822 [PubMed]
- Grossfeld GD, Ginsberg DA, Stein JP, et al. Thrombospondin-1 expression in bladder cancer: association with p53 alterations, tumor angiogenesis, and tumor progression. J Natl Cancer Inst 1997;89:219-27. [Crossref] [PubMed]
- Goddard JC, Sutton CD, Jones JL, et al. Reduced thrombospondin-1 at presentation predicts disease progression in superficial bladder cancer. Eur Urol 2002;42:464-8. [Crossref] [PubMed]
- Canfield AE, Schor AM. Evidence that tenascin and thrombospondin-1 modulate sprouting of endothelial cells. J Cell Sci 1995;108:797-809. [PubMed]
- van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res 2001;305:285-98. [Crossref] [PubMed]
- Liu Z, Wang F, Chen X. Integrin αvβ3-targeted cancer therapy. Drug development research 2008;69:329-39. [Crossref] [PubMed]
- Lityńska A, Przybylo M, Pochec E, et al. Adhesion properties of human bladder cell lines with extracellular matrix components: the role of integrins and glycosylation. Acta Biochim Pol 2002;49:643-50. [PubMed]
- Harabayashi T, Kanai Y, Yamada T, et al. Reduction of integrin beta 4 and enhanced migration on laminin in association with intraepithelial spreading of urinary bladder carcinomas. J Urol 1999;161:1364-71. [Crossref] [PubMed]
- Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer 2010;10:505-14. [Crossref] [PubMed]
- Döme B, Hendrix MJ, Paku S, et al. Alternative vascularization mechanisms in cancer: pathology and therapeutic implications. Am J Pathol 2007;170:1-15. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Santos L, Costa C, Pereira S, et al. Neovascularisation is a prognostic factor of early recurrence in T1/G2 urothelial bladder tumours. Ann Oncol 2003;14:1419-24. [Crossref] [PubMed]
- Goddard JC, Sutton CD, Furness PN, et al. Microvessel density at presentation predicts subsequent muscle invasion in superficial bladder cancer. Clin Cancer Res 2003;9:2583-6. [PubMed]
- Ajili F, Kacem M, Tounsi H, et al. Prognostic impact of angiogenesis in nonmuscle invasive bladder cancer as defined by microvessel density after immunohistochemical staining for CD34. Ultrastruct Pathol 2012;36:336-42. [Crossref] [PubMed]
- Bochner BH, Cote RJ, Weidner N, et al. Angiogenesis in bladder cancer: relationship between microvessel density and tumor prognosis. J Natl Cancer Inst 1995;87:1603-12. [Crossref] [PubMed]
- Raval RR, Lau KW, Tran MG, et al. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol 2005;25:5675-86. [Crossref] [PubMed]
- Mole DR, Maxwell PH, Pugh CW, et al. Regulation of HIF by the von Hippel-Lindau Tumour Suppressor: Implications for Cellular Oxygen Sensing. IUBMB Life 2001;52:43-7. [Crossref] [PubMed]
- Pinto Á, Redondo A, Zamora P, et al. Angiogenesis as a therapeutic target in urothelial carcinoma. Anticancer Drugs 2010;21:890-6. [Crossref] [PubMed]
- Ziello JE, Jovin IS, Huang Y. Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med 2007;80:51-60. [PubMed]
- Niu G, Chen X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr Drug Targets 2010;11:1000-17. [Crossref] [PubMed]
- Neufeld G, Cohen T, Gengrinovitch S, et al. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 1999;13:9-22. [PubMed]
- Koch S, Tugues S, Li X, et al. Signal transduction by vascular endothelial growth factor receptors. Biochemical Journal 2011;437:169-83. [Crossref] [PubMed]
- Crew JP, O'Brien T, Bradburn M, et al. Vascular endothelial growth factor is a predictor of relapse and stage progression in superficial bladder cancer. Cancer Res 1997;57:5281-5. [PubMed]
- Crew JP, O'brien T, Bicknell R, et al. Urinary vascular endothelial growth factor and its correlation with bladder cancer recurrence rates. J Urol 1999;161:799-804. [Crossref] [PubMed]
- Miyake H, Hara I, Yamanaka K, et al. Elevation of serum level of vascular endothelial growth factor as a new predictor of recurrence and disease progression in patients with superficial urothelial cancer. Urology 1999;53:302-7. [Crossref] [PubMed]
- Inoue K, Slaton JW, Davis DW, et al. Treatment of human metastatic transitional cell carcinoma of the bladder in a murine model with the anti-vascular endothelial growth factor receptor monoclonal antibody DC101 and paclitaxel. Clin Cancer Res 2000;6:2635-43. [PubMed]
- Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol 2008;75:346-59. [Crossref] [PubMed]
- Misko A, Ferguson T, Notterpek L. Matrix metalloproteinase mediated degradation of basement membrane proteins in Trembler J neuropathy nerves. J Neurochem 2002;83:885-94. [Crossref] [PubMed]
- Mackay AR, Gomez DE, Cottam DW, et al. Identification of the 72-kDa (MMP-2) and 92-kDa (MMP-9) gelatinase/type IV collagenase in preparations of laminin and Matrigel. Biotechniques 1993;15:1048-51. [PubMed]
- Margulies IM, Höyhtyä M, Evans C, et al. Urinary type IV collagenase: elevated levels are associated with bladder transitional cell carcinoma. Cancer Epidemiol Biomarkers Prev 1992;1:467-74. [PubMed]
- Ozdemir E, Kakehi Y, Okuno H, et al. Role of matrix metalloproteinase-9 in the basement membrane destruction of superficial urothelial carcinomas. J Urol 1999;161:1359-63. [Crossref] [PubMed]
- Gerhards S, Jung K, Koenig F, et al. Excretion of matrix metalloproteinases 2 and 9 in urine is associated with a high stage and grade of bladder carcinoma. Urology 2001;57:675-9. [Crossref] [PubMed]
- Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin Cancer Biol 2010;20:161-8. [Crossref] [PubMed]
- Hara I, Miyake H, Hara S, et al. Significance of matrix metalloproteinases and tissue inhibitors of metalloproteinase expression in the recurrence of superficial transitional cell carcinoma of the bladder. J Urol 2001;165:1769-72. [Crossref] [PubMed]
- Claffey KP, Abrams K, Shih SC, et al. Fibroblast growth factor 2 activation of stromal cell vascular endothelial growth factor expression and angiogenesis. Lab Invest 2001;81:61-75. [Crossref] [PubMed]
- Koch AE, Polverini PJ, Kunkel SL, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992;258:1798-801. [Crossref] [PubMed]
- Huang S, Mills L, Mian B, et al. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am J Pathol 2002;161:125-34. [Crossref] [PubMed]
- Fus ŁP, Górnicka B. Role of angiogenesis in urothelial bladder carcinoma. Cent European J Urol 2016;69:258-263. [PubMed]
- Sobolewski C, Cerella C, Dicato M, et al. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int J Cell Biol 2010;2010:215158
- Takahashi Y, Bucana CD, Liu W, et al. Platelet-derived endothelial cell growth factor in human colon cancer angiogenesis: role of infiltrating cells. J Natl Cancer Inst 1996;88:1146-51. [Crossref] [PubMed]
- Sawase K, Nomata K, Kanetake H, et al. The expression of platelet-derived endothelial cell growth factor in human bladder cancer. Cancer Lett 1998;130:35-41. [Crossref] [PubMed]
- Shimoda M, Mellody KT, Orimo A, et al. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin Cell Dev Biol 2010;21:19-25. [Crossref] [PubMed]
- Franco OE, Shaw AK, Strand DW, et al. Cancer associated fibroblasts in cancer pathogenesis. Semin Cell Dev Biol 2010;21:33-9. [Crossref] [PubMed]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009;9:162-74. [Crossref] [PubMed]
- Raychaudhuri B, Rayman P, Ireland J, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol 2011;13:591-9. [Crossref] [PubMed]
- Solinas G, Germano G, Mantovani A, et al. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 2009;86:1065-73. [Crossref] [PubMed]
- Burkholder B, Huang RY, Burgess R, et al. Tumor-induced perturbations of cytokines and immune cell networks. Biochim Biophys Acta 2014;1845:182-201.
- Ma J, Liu L, Che G, et al. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer 2010;10:112. [Crossref] [PubMed]
- Leek RD, Harris AL. Tumor-associated macrophages in breast cancer. Journal of mammary gland biology and neoplasia 2002;7:177-89. [Crossref] [PubMed]
- Hanada T, Nakagawa M, Emoto A, et al. Prognostic value of tumor-associated macrophage count in human bladder cancer. Int J Urol 2000;7:263-9. [Crossref] [PubMed]
- Takayama H, Nishimura K, Tsujimura A, et al. Increased infiltration of tumor associated macrophages is associated with poor prognosis of bladder carcinoma in situ after intravesical bacillus Calmette-Guerin instillation. J Urol 2009;181:1894-900. [Crossref] [PubMed]
- Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-β:“N1” versus “N2” TAN. Cancer cell 2009;16:183-94. [Crossref] [PubMed]
- Galdiero MR, Bonavita E, Barajon I, et al. Tumor associated macrophages and neutrophils in cancer. Immunobiology 2013;218:1402-10. [Crossref] [PubMed]
- Tanchot C, Terme M, Pere H, et al. Tumor-infiltrating regulatory T cells: phenotype, role, mechanism of expansion in situ and clinical significance. Cancer Microenviron 2013;6:147-57. [Crossref] [PubMed]
- Mougiakakos D, Choudhury A, Lladser A, et al. Regulatory T cells in cancer. Adv Cancer Res 2010;107:57-117. [Crossref] [PubMed]
- Kim R, Emi M, Tanabe K, et al. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res 2006;66:5527-36. [Crossref] [PubMed]
- Chopin D, Barei-Moniri R, Maillé P, et al. Human urinary bladder transitional cell carcinomas acquire the functional Fas ligand during tumor progression. Am J Pathol 2003;162:1139-49. [Crossref] [PubMed]
- Gupta GP, Massagué J. Platelets and metastasis revisited: a novel fatty link. J Clin Invest 2004;114:1691-3. [Crossref] [PubMed]
- Singh S, Sadanandam A, Singh RK. Chemokines in tumor angiogenesis and metastasis. Cancer Metastasis Rev 2007;26:453-67. [Crossref] [PubMed]
- Kawanishi H, Matsui Y, Ito M, et al. Secreted CXCL1 is a potential mediator and marker of the tumor invasion of bladder cancer. Clin Cancer Res 2008;14:2579-87. [Crossref] [PubMed]
- Sun X, Cheng G, Hao M, et al. CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev 2010;29:709-22. [Crossref] [PubMed]
- Kalluri R. The biology and function of exosomes in cancer. J Clin Invest 2016;126:1208-15. [Crossref] [PubMed]
- Welton JL. Exosomes: a source of novel disease biomarkers in bladder cancer: Cardiff: Cardiff University; 2010.
- Chaudhary U, Golshayan A, Brisendine A, et al. Phase II trial of neoadjuvant cisplatin, gemcitabine, and bevacizumab followed by radical cystectomy (RC) in patients with muscle-invasive transitional cell carcinoma (TCC) of the bladder. J Clin Oncol 2011;29:276. [Crossref]
- Siefker-Radtke A, Millikan R, Kamat A, et al. A phase II trial of sequential neoadjuvant chemotherapy with ifosfamide, doxorubicin, and gemcitabine (IAG), followed by cisplatin, gemcitabine and ifosfamide (CGI) in locally advanced urothelial cancer (UC): Final results from the MD Anderson Cancer Center. J Clin Oncol 2008;26:5079. [Crossref]