
Normal tissue tolerance
Introduction
Normal tissue radiation tolerance refers to the response of previously unirradiated tissues to a variety of very different exposure scenarios: total or large partial body exposure with ‘high’ doses in an ‘acute’ manner, e.g., in radiation accidents, or in a more ‘chronic’ way, e.g., through close and permanent contact with radioactive sources. A more localized ‘high’ dose, conformed to specific target structures, is achieved with modern radiotherapy techniques. In contrast, concentration of ‘low’ radiation doses is usually induced with most diagnostic procedures that involve ionizing radiation; however, the latter is also imminent in radiotherapy outside the high-dose structures. The present overview will not include the response of highly sensitive tissues (e.g., the eye lens, gonads, embryo/fetus). Also, the tolerance of normal tissues to radiation induction or promotion of tumours will not be covered. This present review rather focuses on the standard OAR in radiotherapy.
Pathobiology of normal tissue radiation effects
Early (acute) normal tissue reactions are for the first time diagnosed during or shortly after a course of radiotherapy. In contrast, late (chronic) radiation responses usually become clinically manifest after long latent times of months to many years. General, specific (radio)biological characteristics can be found for early and late effects (1), which will be summarized below.
Early radiation effects
Typically, tissues with a high proliferative activity, such as bone marrow, epidermis, or mucosae of the gastro-intestinal tract, display early radiation effects. In these so-called ‘turnover’ tissues, ongoing cell production counteracts a permanent physiological cell loss, e.g., by mechanical stress at surface epithelia. Symptoms of radiation exposure are based on the impairment of proliferation, i.e., the dose-dependent sterilization of epithelial stem cells, while cell loss is largely independent of the radiation exposure over wide dose ranges, and continues at its physiological rate. This imbalance between cell production and cell loss results in progressive hypoplasia and eventually complete loss of functional cells. Early effects are regularly accompanied by vascular and inflammatory reactions (1).
The time course of early radiation reactions is schematically presented in Figure 1. The latent time for early radiation responses is largely independent of the radiation dose and is mainly defined by the overall turnover time, i.e., the time in which all cells would physiologically be renewed once by (stem) cell proliferation. Restoration of early effects, based on proliferation of surviving cells, is usually complete. Importantly, in contrast to latent times, the time to healing depends on dose, as less stem cells survive the treatment (1).
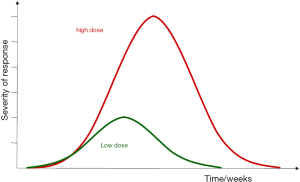
It must be noted that in some organs, early radiation effects may develop independently of radiation-induced hypoplasia but related to other biological mechanisms, which are less well studied, such as inflammatory or neurovegetative disturbances such as brain oedema, pneumonitis or diarrhea.
Late radiation sequelae
Late radiation effects can be observed in virtually all tissues and organs. The pathomechanisms are more complex than those of early reactions (1-3). Radiation-induced changes are seen in the parenchyma of the organs, i.e., in the tissue specific compartments, but also in the connective and vascular tissue. Moreover, the immune system (macrophages, mast cells) significantly contributes to the tissue reactions.
Late radiation effects are usually irreversible, and potentially progressive over long time periods. Moreover, their latent time inversely and their progression rates directly depend on the radiation dose, as indicated in Figure 2. Advanced radiotherapy techniques are mostly associated with an increase in the subvolumes of organs at risk (OAR) that receive low doses and are associated with significant dose inhomogeneities. Hence, characterisation of the treatment-induced morbidity profile of (new) radiotherapy protocols requires very long follow-up intervals, associated with a precise assessment and documentation of the complication endpoints. The risk for a manifestation of a chronic radiation reaction can essentially remain for the entire life of the patient (4).
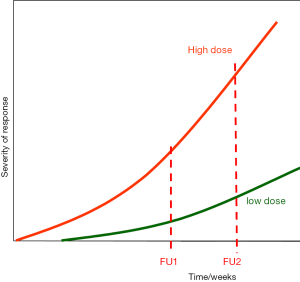
Whereas for early radiation effects, stem cell sterilization plays an important role, this radiobiological mechanism is of small or no importance in the pathogenesis of most late radiation effects. Here, besides parenchymal cell functional changes, further tissue structures and cell populations are involved. These include vascular endothelial cells, mainly in small blood vessels and capillaries, where irradiation initially induces functional changes (1-3). Foci of endothelial detachment are regularly seen, accompanied by leukocyte adhesion and infiltration, and migration of serum components into the vessel wall and subendothelial structures. Moreover, thrombus formation and capillary occlusion are frequently observed (5). Eventually, a progressive loss of capillaries occurs. Moreover, telangiectasia is a further form of the vascular response to irradiation. The latter can be a cosmetic problem (skin), but also have a clear tendency for direct or indirect (secondary ulceration) bleeding (e.g., intestine, urinary tract, CNS) and thus a clinical impact. Radiation also causes mitotic fibroblasts to differentiate, which significantly increases collagen synthesis and deposition into the surrounding tissue, eventually resulting in fibrosis (1,6,7). The orchestrated interaction of changes in the individual components of the late responding tissues results in progressive parenchymal damage and eventually in loss of function within the irradiated volume (IV). However, the clinical consequences are dependent on the individual tissue and organ architecture and the exposed volume (see below).
Consequential late effects (CLE)
In general, the risk for late effects is independent of the severity, i.e., intensity or duration, of early reactions. Yet, for some organs, interactions between early and chronic sequelae are known, which can result in CLE. This is the case, where the early responding tissue compartment (e.g., epithelium) has a protective function against mechanical and/or chemical stress, which is impaired during the early radiation response. Consequently, secondary traumata can impact on the target structures of the late sequelae (1,3,8). CLEs are observed in intestine, urinary tract, oral mucosa and particularly stressed skin localizations (1,8).
Assessment and documentation of normal tissue side effects
For assessment, documentation and quantitation of radiotherapy complications, the frequency of examinations and the scoring system applied clearly needs to be adjusted to the response studied. In early responding tissues, significant changes can occur within short time intervals of even a few days. Hence, their detailed documentation requires evaluation at least on a weekly basis, preferably 2–3 times per week. In contrast, late effects may be scored in intervals of several months after the end of radiotherapy, and even in annual or longer intervals thereafter; the precise timing should be based on the panel of complications that is expected. For some late effects, e.g., in heart, kidney or urinary bladder, the time to clinical manifestation can last for decades. Hence, life-long follow-up of the patients is recommended. It must be emphasized that some late endpoints show compensation or healing (9-11), which must be considered in the analysis of complication rates (12).
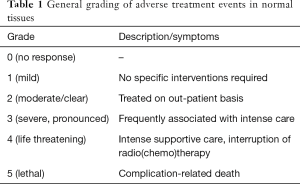
A number of standardized classification systems have been established, where complications are usually graded from 0 to 5 (Table 1). It must be emphasized, however, that these general scoring criteria should assist the radio-oncologist in the characterization of the severity of an adverse event. For example, a grade 4 reaction of the skin has consequences that are completely different from a grade 4 reaction in the lung.
Full table
The most widely used classification systems with their sources are listed in Table 2. Besides these systems, more detailed protocols for dedicated clinical studies or designed for certain organs have been proposed (1,13). In principle, a documentation system for all individual organs in all institutions involved in follow-up of patients would be desirable. Yet, for inter-institutional comparisons, the scoring procedures definitely require harmonization.

Full table
The R’s of (normal tissue) radiobiology
In 1975, Withers introduced 4 R’s of radiotherapy as treatment-related factors that significantly influence outcome and complications (14): recovery (fractionation effect), repopulation (effect of overall treatment time), redistribution (cell cycle effects) and reoxygenation (oxygen effect). From these, only recovery and repopulation are relevant for normal tissues. Steel (14) introduced the intrinsic radiosensitivity as a further, 5th R. Tissue radiation tolerance (to a single radiation exposure) represents a composite of the intrinsic radiosensitivity of all target components and the respective target cell numbers. The improved conformation of the high dose volume to the tumor volume resulted in an increasing inhomogeneity of the radiation dose within OAR. Therefore, the i’R’rradiated volume was suggested as a 6th R into the list (3,15).
Intrinsic radiosensitivity
According to the target/stem cell concept, the radiation tolerance of any organ or tissue is defined by the number and the intrinsic sensitivity of the tissue specific target cells. Predominantly for late complications, this is a combination of the sensitivities of the individual cell populations that contribute to the tissue radiation response. For most normal tissues, there are no specific markers for stem cells, and hence this must still be considered a hypothetical—but mostly valid—concept. Yet, it needs to be stressed that target cells must not necessarily be stem cells. Whenever structural radiation damage is of clinical importance, particularly for late radiation sequelae, the target cell concept is highly speculative. Target structure radiosensitivity in general may not be related to cellular radiosensitivity of individual target or stem cells, but also to the biological interaction of different target/stem cell populations.
Recovery—the fractionation effect
Basically, administration of a total dose in fractions results in an increase in isoeffective doses, or a decrease of morbidity at a given total dose. For various normal tissues, the fractionation effect has been quantitated in numerous preclinical and clinical studies over the last decades. The current knowledge of relationships between tolerance dose and dose per fraction for early and chronic complications, and tumour response, are the basis for optimization of radiotherapy protocols with regard to dose per fraction and total dose. In general, it is assumed that the fractionation effect is low—but still significant—for early normal tissue reactions, and pronounced for late reactions.
The linear-quadratic (LQ) model
The LQ model (16-19), can be applied to describe the relationship between total isoeffective doses and dose per fraction and thus to get estimates of effectivity/toxicity after changes in dose per fraction and total dose (16,20-22). Here, a certain effect E in a normal tissue after irradiation with a total dose D is described by a linear term with the value α/β, i.e.,
The α/β-value describes the range, over which dose-effect curves are shifted to higher isoeffective doses with a decrease in dose per fraction, corresponding to an increase in number of fractions. A low α/β-value represents a substantial increase in tolerance with a reduction in dose per fraction, while a high α/β-value reflects a minor fractionation effect.
It has to be stressed that the LQ model at the tissue or endpoint level is not based on radiobiological mechanisms, such as target cell survival, but is nothing as a mathematical fit of the change of dose effect curves or the incidence of treatment adverse effects, if doses per fraction doses are modified (either by hypo- or hyper-fractionation or by changes in dose distribution within the IV). In general, however, most late radiation endpoints are more sensitive to changes in dose per fraction, as indicated by their low α/β-value (<6 Gy), while most early (and thus also consequential late) endpoints display a still significant, but low fractionation sensitivity (20-22). Hence, (some) late normal tissues endpoints can selectively be spared by lowering the dose per fraction (hyper-fractionation); others, less dominating and hence of less influence on the general estimate of OAR radiosensitivity in clinical studies, may not follow this rule. In contrast, high doses per fraction, e.g., applied in stereotactic treatments, aggravate late effects, and hence must be administered in a highly conformal manner. The α/β-values for various animal and human normal tissues have been summarized [e.g., (1,20-22)], but can also be found in the reports of the QUANTEC initiative (International Journal of Radiation Oncology Biology Physics 76 Suppl., 2010).
The LQ model allows for comparison of the (equi)effectivity of different fractionation protocols, e.g., by transformation of a given schedule (fraction size x, total dose Dx) into the equi-effective total dose in 2 Gy fractions (EQD2), by using the above mentioned formulae with d=2 Gy and D=EQD2.
This also has the advantage that sub-protocols with different fractionation schedules can be added to a total equi-effective dose. An alternative method (18) calculates the biologically equivalent dose, BED (not to be confused with the equivalent dose used in radiation protection!).
With the LQ-approach, extrapolations for very substantial deviations from 2 Gy/fraction must be handled critically. At doses per fraction <1.0 Gy, low-dose hyper-radiosensitivity (23,24) might result in underestimation of the biological effect of a given total dose. Also, the biological effect of high doses per fraction (>6–10 Gy), e.g., applied in stereotactic treatments or brachytherapy) may be overestimated by the LQ-model (25).
Incomplete recovery
Recovery follows a certain dynamics, which is usually characterized as mono-exponential or bi-exponential (21). Recovery kinetics can hence be described by recovery halftimes, i.e., the time intervals, over which 50% of the recoverable damage has been restored. These halftimes have been repeatedly summarized (20-22).
Incomplete recovery decreases the tissue tolerance, which must be taken into account in treatment protocol decisions, e.g., with multiple fractions per day. For this, the incomplete recovery model as suggested by Joiner and Bentzen (20) or Thames (21) can be applied.
Recovery kinetics is also of importance in scenarios of continuous exposure, either in low dose-rate brachytherapy or in radionuclide therapy. There, recovery occurs already during the administration. The respective formalisms and equations are illustrated e.g., in Van der Kogel (26) or Dale (27). For administration of radionuclides, in addition to the exposure time, also the decrease in dose rate based on the physical halftime of the nuclide, and also the decrease in the dose rate through excretion of the radionuclide, i.e., the biological halftime, must be taken into consideration (28,29).
Repopulation—the effect of overall treatment time
Clinical and experimental observations
Repopulation is defined as an increase in radiation tolerance with increasing overall treatment time (29). This is observed for typical early radiation effects, based on their regenerative response. The biological mechanisms are complex, and include a profound restructuring of the proliferative organization, both at the stem/target cell and the total tissue level. The clinical and experimental observations illustrating the repopulation effect are described in detail in Dörr (29).
Clinical studies with accelerated radiotherapy protocols (shortened overall treatment time, increased weekly dose) in head-and-neck cancer, have focused on oral mucositis as the dose-limiting early complication. Consistently, an aggravation was observed (30,31). Repopulation processes start within the first weeks of radiotherapy in patients (32,33) and preclinical models (34-36).
The capacity of repopulation (dose compensation), once these processes have started, was estimated for human oral mucosa to be in the range of 0.5–1.0 fractions per day that are counteracted (36,37).
Mechanisms of repopulation in normal tissues
All clinical and experimental studies yielded three consistent observations (35,36,38):
- The radiation effect is counteracted with increasing overall treatment time, once repopulation has become effective, i.e., 1–2 weeks in oral mucosa, 3–4 weeks in other epithelia;
- The dose, which is compensated due to repopulation processes in normal tissues, is in the range of up to 5×2 Gy/week, as e.g., indicated by healing of mucosal ulcers in the last treatment weeks (39,40), and;
- The rate of overall tissue cell depletion per unit dose is significantly lower after as compared to before the onset of repopulation.
The underlying mechanisms have been summarized as the 3 A’s, which represent a significant restructuring of the proliferative organization of the turnover tissues: ‘A’symmetry loss and ‘A’cceleration of stem cell divisions, and ‘A’bortive divisions of already doomed cells (34-38).
Volume effects
Volumes for the therapeutic administration of ionising radiation have been defined by the International Commission on Radiation Units, ICRU (41). Normal tissue components are included in all of them. The gross tumour volume (GTV) contains normal tissue elements, e.g., vasculoconnective tissue. The clinical target volume (CTV) and planning target volume (PTV) encompass a relevant number of normal parenchymal cells of the respective organ. The additional volume extending the CTV/PTV to the treated volume (TV), i.e., the volume covered by the clinically effective dose, is exclusively composed of normal tissue. Nearly always, the PTV contains more normal tissue than tumour tissue.
The IV that receives a dose which must be regarded as significant in relation to the induction of clinical OAR endpoints is mainly dependent on physical treatment parameters, such as radiation quality, kind of radiotherapy (brachytherapy, conformal teletherapy, intensity modulated radiotherapy, stereotactic radiotherapy), treatment planning (number of fields, etc.). The progress in radiation physics during the last decades has resulted in significant and progressive conformation of the TV to the PTV and of the IV to the TV, associated with a significant decrease in the volumes of normal tissues exposed to significant, ‘high’ doses and thus in the incidence of (early) adverse events. This, however, does not solve the clinical problem, as it needs to be emphasized that the high conformality is associated with substantial inhomogeneities in the dose distribution within these volumes, and—even more important—in an increase in volumes exposed to lower doses. In consequence, the effect of the fractional volume of an OAR exposed to certain, even low doses, gains increasing importance. Today, the I’R’radiated volume of an organ must be considered the “6th R” of radiotherapy (15).
The general principles of the ‘volume effect’ in normal tissues have been extensively reviewed e.g., by Dörr and Van der Kogel (42) or in the reports of the QUANTEC initiative (International Journal of Radiation Oncology Biology Physics 76 Suppl., 2010). These will be briefly summarized in the following sections.
Tissue architecture and volume effects
Functional subunits (FSU) represent a radiobiological concept to explain the response of normal tissues to varying volumes of radiation exposure (2,3,15,43). A FSU was originally defined as the largest tissue subvolume that can be regenerated from a single surviving stem cell. Irradiation primarily damages FSU independent of each other. However, the clinical consequences are depending on the arrangement of the FSU within the exposed organ: The FSU can be arranged either in parallel or in a series.
In organs with a serial (tubular) arrangement of their FSU, inactivation of only one FSU theoretically results in clinical side effects in the downstream compartments of the organ, mostly in a binary response. In such organs, the risk of clinical symptoms is highly related to dose “hot spots”, while the dose distribution within the remainder of the tissue is less relevant, if the dose is below the FSU tolerance (3,15). Examples for (mainly) serially organized organs are spinal cord, intestine and esophagus.
In tissues with a parallel organization, the FSU are functioning independently and thus clinical consequences of their damage are only observed, if their number decreases to a certain, endpoint-specific threshold. Here, radiation exposure should be adjusted to a threshold organ volume; within this volume, larger doses may be administered (15). Examples for (predominantly) parallel organs are lung, kidney, and liver. However, it needs to be stressed that no organ is structured simply as a batch (parallel organization) or a chain (serial organization) of FSU. For example, in organs that are classified as parallel, the vasculature, at least larger vessels, must be regarded as a serial tissue component.
Volume effect and normal tissue complication probability (NTCP) models
Based on the above mentioned considerations, the concept of a certain degree of seriality of organs has been introduced into mathematical models of the volume effects [e.g., (44)]. This concept is useful in the explanation of the relative radiosensitivity of some organs, like kidney and lung, can compensate for destruction of large volumes without loss of function, whereas in other tissues generally considered as relatively radioresistant such as spinal cord, radiation damage within only a small volume can result in severe clinical consequences.
Several further NTCP models have been suggested for inhomogeneous dose distributions (45,46). The resulting Lyman-Kutcher-Burman (LKB) model and its modifications is currently presumably one of the most commonly used models for predicting normal tissue complication probability. Yet, it has to be stressed that endpoints and symptoms of late radiation damage do not occur in tissue “volumes” but in specific, sensitive structures, and that radiation damage to different substructures in the same organ leads to different pathophysiological endpoints and symptoms of late normal tissue damage such as in the heart, where—depending on the dose distribution within the organ—coronary infarction or myocardial insufficiency may the dominant late normal tissue endpoint. Moreover, the clinical consequences of exposure of different substructures of ‘a’ normal tissue may result in substantially different clinical consequences, for example irradiation of a certain area of oral mucosa at the floor of the mouth or at the lips, where the latter leads to a significant impact on the patient’s quality of life in the early phase, but also to substantial CLEs.
Mathematical models for the estimation of NTCP, however, do not take into account the influence of cellular migration and regeneration from outside the irradiated area/volume. Moreover, they do not include other factors, like regional differences in radiation sensitivity within one organ that have been demonstrated in lung, urinary bladder or parotid gland. Moreover, interactions of radiation effects between ‘corresponding, functionally dependent’ organs, such as heart and lung or liver, are not taken into consideration.
Importantly, NTCP models also do not consider the functional status of the non-irradiated organ volume. Lung function parameters in heavy smokers may be substantially impaired, and the usually applied dose constraints may clearly overestimate the tolerance of the total lung. Moreover, previous or additional chemotherapy may impact on the function of the un-irradiated organ volume. Therefore, the functional status of unirradiated parts of tissues and organs must be assessed and taken into account for the definition of tolerance limits for radiotherapy in the individual patient. This must be based on the experience and expert knowledge of the responsible radiation oncologist.
The available NTCP models, which are progressively integrated into treatment planning systems, should therefore be used with great caution and with clear consideration of all their pitfalls and drawbacks. They should be regarded as an aid to the evaluation and comparison of clinical data using different treatment set-ups, rather than giving accurate predictions of clinical outcome (15).
QUANTEC—quantitative analysis of normal tissue effects in the clinic
All NTCP models are based on estimates of tissue-specific tolerance doses and fractionation parameters, like the α/β-value and halftimes for recovery of sublethal damage. For tolerance doses, the compilation by Emami et al. (46) is still frequently cited, which was an excellent initiative in defining organ (rather than endpoint-related) tolerance doses. However, this was based on “opinions and experience of the clinicians from four universities” (47), and hence required validation. QUANTEC, the initiative on Quantitative Analysis of Normal Tissue Effects in the Clinic (in the reports of the QUANTEC initiative (International Journal of Radiation Oncology Biology Physics 76 Suppl., 2010) provides focused summaries of the dose/volume/outcome information for many, though not all organs and tissues (48). The organ specific QUANTEC papers include—amongst other topics—an appreciation of the clinical significance of various endpoints, review the currently available dose-volume tolerance information and factors affecting risk, and recommend, where possible, dose-volume constraints. More recently, further reports on specific tissue and organ tolerances have been published, which cannot comprehensively be summarized in this chapter. The interested reader is strongly advised to follow the current literature. It also needs to be emphasized here, however, that tolerance doses (in terms of EQDx rather than absorbed dose as frequently used in dose-volume histograms) need to be defined for individual endpoints, rather than OAR in general.
Principles for the mitigation of normal tissue complications
In this section, the principal approaches for biological response modification, which are based on preclinical (animal) models or in first clinical trials, which have recently been described in Dörr (48), are briefly summarized and updated. General supportive care strategies, adapted to good clinical practice, which are described in various guidelines, will not be discussed. Moreover, neither in vitro studies, nor investigations with exposure to very large single doses, without proof of applicability in a clinical situation, will be considered.
At a NCI workshop on normal tissue protection (49), it was recommended to describe interventions in the development of radiation effects with regard to their timing as illustrated in Table 3. With regard to radiotherapy, the respective terminology needs to be slightly modified, as prophylactic approaches include interventions before exposure, but also until the threshold tolerance dose of a certain endpoint is reached during a series of fractionated irradiations. Hence, there is an overlap between prophylaxis, defined clinically, and mitigation, in terms of interaction with early processes at a molecular level. Prevention is a term frequently used to describe interventions that are applied before the onset of clinical symptoms, and hence refers to prophylaxis as well as mitigation (50).

Full table
One major prerequisite for the reasonable clinical application of normal tissue response modifiers is the association with a therapeutic gain. This must be based on exclusion of tumour protective effects, or on a relatively greater effect on the specific normal tissue investigated compared to tumours. This analysis usually requires preclinical studies in experimental animals, analyzing clinically relevant endpoints for both tumour and normal tissue effects. With regard to irradiation protocols, single dose administration may be considered to reflect stereotactic, intraoperative or brachytherapy treatments with relatively large doses. However, for the representation of standard, external-beam radiotherapy, protection and mitigation strategies must be tested with fractionation protocols that reflect the routine clinical situation as close as possible, i.e., including daily fractions with doses in the clinical range, administered over several weeks. The latter is essential e.g., to analyze potential interactions, beneficial or counterproductive, with repopulation processes in both tumours and normal tissues.
Local irradiation of the normal tissues as well as of the tumours is preferred for these investigations, as total or large volume partial body radiation exposure can significantly alter organ responses with regard to quality of the pathophysiology and -morphology, or even the fractionation effect, e.g., through effects on the immune system. This has clearly been demonstrated for the radiation response of the lung, where the fibrosis pattern changes, and a very low fractionation sensitivity is observed after conditioning treatment for stem/progenitor cell transplantation. Such studies may apply to accidental radiation exposure or intended radiological attacks, or to conditioning irradiation for the treatment of hematological malignancies.
The cascade of events, here depicted as “damage processing”, after radiation exposure (Figure 3) starts from the initial induction of free radicals and acute oxidative stress, which result in the modification of the activity of transcription factors, and in consequence in the modulation of a variety of intracellular and extracellular signaling chains. These changes occur not only in parenchymal cells, but also in the other tissue components (fibroblasts, endothelial cells). Moreover, activation of immune cells, mainly macrophages, directly by irradiation or attracted by the above mentioned changes (e.g., increased ICAM-1 expression) contributes to the radiation-induced processes in normal tissues. The combination of these events induces unspecific and tissue specific changes at the cellular/histological level, such as (mainly mitotic) cell death, DNA repair, chronic oxidative stress, differentiation of fibroblasts and parenchymal cells, proliferation of cells in early responding tissues, and many others. This orchestrated response then leads to the known tissular and clinical changes described above.
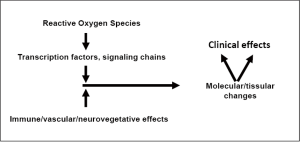
Interventions in the damage processing events can in principle be performed at each of the levels of the cascade illustrated in Figure 3. This has been summarized by Dörr (48). It has to be noted, that some interventional strategies are specific for certain endpoints, either early or late; in this aspect, however, the relevance of amelioration of an early radiation response for the manifestation of CLEs also deserves consideration. Some prominent principles and examples of damage processing modulation will be briefly reviewed in this section.
In the vast majority of normal tissues, the partial pressure of oxygen is clearly above the range where the oxygen effect, i.e., a reduction in cellular/tissue radiosensitivity, is important (51). Therefore, any reduction in oxygen levels, i.e., by induction of hypoxia, could have a potential to increase tissue tolerance. However, systemic hypoxia, e.g., by breathing air with a reduced oxygen concentration, will likely be associated with an increase in the fraction of hypoxic cells within the tumour, and therefore lead to increased tumour radioresistance, which should be avoided.
The use of radical scavenging drugs or the stimulation of endogenous detoxification mechanisms has been proposed to reduce effects of acute oxidative stress. Clinical studies have been performed on the effect of various anti-oxidants in combination with radiotherapy, with controversial results reviewed by Dörr (48).
Exogenous growth factors may be applied in order to activate or stimulate tissue-specific endogenous signaling cascades. Alternatively, radiation-induced changes in signaling activity may be achieved by either antibodies against the growth factor or the respective receptors, or by downstream interaction, e.g., by receptor tyrosine kinase inhibitors. Irradiation results in an upregulation of endogenous growth factors (e.g., TNF-alpha) and/or their receptors (e.g., EGFR). These endogenous processes may also be targeted.
Anti-inflammatory approaches are frequently applied as symptomatic, supportive treatment in order to manage edema and pain associated with early inflammation. However, no conclusive results are available for these approaches with regard to prevention of (late) radiotherapy side effects. At least, an impact on CLE, such as mandibular osteoradionecrosis as a consequence of oral mucositis in head-and-neck
The relevance of macrophage responses for normal tissue side effects is discussed controversially. For late effects, e.g., in lung, intestine or urinary bladder a contribution of alveolar macrophages to the orchestrated reaction of the tissue has been demonstrated (1-3,7,52). Also, for early radiation side effects, changes in macrophage activation have been observed, e.g., (53). Low-level laser treatment may also induce an anti-inflammatory effect (54,55).
The severity of early radiation effects during fractionated irradiation is clearly related to the regeneration response of the tissue, depicted as repopulation. Exogenous stimulation of cell production in epithelial tissues has hence been suggested to reduce early side effects (3,38,50).
For late effects in normal tissues, a long-lasting perpetuation of the production of reactive oxygen and nitrogen species appears to play an essential role (3,6,7,52). Strategies for the reduction of the chronic oxidative stress cascades have been tested for fibrotic changes in skin, using a combination of pentoxifylline (PTX) and tocopherol (vitamine E) as anti-oxidative agents; the results of clinical trials, however, remain controversial.
An innovative approach for the amelioration of normal tissue radiation effects is (adult) stem cells therapy. This includes the administration of bone marrow (i.e., haematopoietic plus mesenchymal stem cells) or mesenchymal stem cells, or the mobilization of autologous (bone marrow) stem cells by growth factors, e.g., granulocyte colony stimulating factor (G-CSF). These strategies have been successfully tested in preclinical models of radiation injury in skin, salivary glands, intestine and oral mucosa, as reviewed by Dörr and Schmidt (3).
In conclusion, a variety of strategies for the prophylaxis, mitigation or treatment of radiation side effects have been suggested, based on the biology of the response of the specific tissues, or the pathomechanisms of individual endpoints to irradiation. It must be emphasized, however, that currently most of these interventions are experimental, although some in advanced preclinical studies. Only a few approaches have so far been translated into clinical studies. In the near future, none of these biology-based strategies will result in a reduction of adverse treatment effects to a clinically irrelevant level. Hence, it remains in the hands of the radiation oncologist to reduce these complications to an acceptable level—through adequate spatial (volume effects) and temporal (recovery, repopulation) designs of the treatment protocols and hence to achieve a further therapeutic gain.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Durante, Giusi I. Forte, Giorgio Russo) for the series “Radiobiological models towards a personalized radiation oncology” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.06.45). The series “Radiobiological models towards a personalized radiation oncology” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dörr W. Chapter 13: Pathogenesis of normal tissue side effects. In: Joiner M, Van der Kogel A. editors. Basic Clinical Radiobiology, 4th Edition. London: Hodder Arnold, 2009:169-90.
- Dörr W. The radiobiology of tissue reactions. Ann ICRP 2015;44:58-68. [Crossref] [PubMed]
- Dörr W, Schmidt M. Chapter 7.05: Normal tissue radiobiology. In: Hendry JH. editor. Comprehensive Biomedical Physics. Amsterdam-Oxford-Waltham: Elsevier, 2014;7:75-95.
- Jung H, Beck-Bornholdt HP, Svoboda V, et al. Quantification of late complications after radiation therapy. Radiother Oncol 2001;61:233-46. [Crossref] [PubMed]
- Fajardo LF, Berthrong M, Anderson RE. Radiation Pathology. New York: Oxford University Press, 2001.
- Rodemann HP, Bamberg M. Cellular basis of radiation-induced fibrosis. Radiother Oncol 1995;35:83-90. [Crossref] [PubMed]
- Yarnold J, Brotons MC. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol 2010;97:149-61. [Crossref] [PubMed]
- Dörr W, Hendry JH. Consequential late effects in normal tissues. Radiother Oncol 2001;61:223-31. [Crossref] [PubMed]
- Goldner G, Pötter R, Kranz A, et al. Healing of late endoscopic changes in the rectum between 12 and 65 months after external beam radiotherapy. Strahlenther Onkol 2011;187:202-5. [Crossref] [PubMed]
- Stock M, Dörr W, Stromberger C, et al. Investigations on parotid gland recovery after IMRT in head and neck tumor patients. Strahlenther Onkol 2010;186:665-71. [Crossref] [PubMed]
- Schmid MP, Potter R, Bombosch V, et al. Late gastrointestinal and urogenital side-effects after radiotherapy – incidence and prevalence. Subgroup-analysis within the prospective Austrian-German phase II multicenter trial for localized prostate cancer. Radiother Oncol 2012;104:114-8. [Crossref] [PubMed]
- Bentzen SM, Dörr W, Anscher M, et al. Normal Tissue effects: Reporting and Analysis. Semin Radiat Oncol 2003;13:189-202. [Crossref] [PubMed]
- Withers HR. The four R’s of radiotherapy. In: Lett JT, Adler H. editors. Advances in Radiation Biology, 5th edn, Academic Press, New York, 1975;241-71.
- Steel GG. Basic Clinical Radiobiology for Radiation Oncologists. London-Boston-Sydney-Auckland: Edward Arnold, 1993.
- Bentzen SM, Dörr W, Gahbauer R, et al. Bioeffect modeling and equieffective dose concepts in radiation oncology – Terminology, quantities and units. Radiother Oncol 2012;105:266-8. [Crossref] [PubMed]
- Douglas BG, Fowler JF. The effect of multiple small doses of X rays on skin reactions in the mouse and a basic interpretation. Radiat Res 1976;66:401-26. [Crossref] [PubMed]
- Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. British Journal of Radiology 1989;62:679-94. [Crossref] [PubMed]
- Thames HD Jr, Withers HR, Peters LJ. Tissue repair capacity and repair kinetics deduced from multifractionated or continuous irradiation regimens with incomplete repair. Br J Cancer Suppl 1984;6:263-9. [PubMed]
- Bentzen SM, Joiner MC. The linear-quadratic approach in clinical practice. In: Joiner M, Van der Kogel A. editors. Basic clinical radiobiology, 4th ed. London: Hodder Arnold, 2009;120-34.
- Joiner MC, Bentzen SM. Fractionation: The linear-quadratic approach. In: Joiner M, Van der Kogel A. editors. Basic Clinical Radiobiology, 4th ed. London: Hodder Arnold, 2009;102-19.
- Thames HD, Hendry JH. Fractionation in Radiotherapy. Taylor and Francis, London-New York-Philadelphia, 1987.
- Joiner MC, Marples B, Lambin P, et al. Low-dose hypersensitivity: Current status and possible mechanisms. Int J Radiat Oncol Biol Phys 2001;49:379-89. [Crossref] [PubMed]
- Marples B, Lambin P, Skov KA, et al. Low dose hyper-radiosensitivity and increased radioresistance in mammalian cells. Int J Radiat Biol 1997;71:721-35. [Crossref] [PubMed]
- Joiner MC. Quantifying cell kill and cell survival. In: Joiner M, Van der Kogel A. editors. Basic Clinical Radiobiology, 4th ed. London: Hodder Arnold, 2009:48-55.
- Thames HD. An 'incomplete-repair' model for survival after fractionated and continuous irradiations. Int J Radiat Biol Relat Stud Phys Chem Med 1985;47:319-39. [Crossref] [PubMed]
- Van der Kogel AJ. The dose-rate effect. In: Joiner M, Van der Kogel A. editors. Basic Clinical Radiobiology, 4th ed. London: Hodder Arnold, 2009:158-68.
- Dale RG. The application of the linear-quadratic dose-effect equation to fractionated and protracted radiotherapy. Br J Radiol 1985;58:515-28. [Crossref] [PubMed]
- Millar WT. Application of the linear-quadratic model with incomplete repair to radionuclide directed therapy. Br J Radiol 1991;64:242-51. [Crossref] [PubMed]
- Dörr W. Time factors in normal tissue responses to irradiation. In: Joiner M, Van der Kogel A. editors. Basic Clinical Radiobiology, 4th ed. London: Hodder Arnold, 2009;149-57.
- Horiot JC, Bontemps P, van den Bogaert W, et al. Accelerated fractionation (AF) compared to conventional fractionation (CF) improves locoregional control in the radiotherapy of advanced head and neck cancers: Results of the EORTC 22851 randomized trial. Radiother Oncol 1997;44:111-21. [Crossref] [PubMed]
- Dörr W, Hamilton C, Boyd T, et al. Radiation-induced changes in cellularity and proliferation in human oral mucosa. Int J Radiat Oncol Biol Phys 2002;52:911-7. [Crossref] [PubMed]
- Maciejewski B, Zajusz A, Pilecki B, et al. Acute mucositis in the stimulated oral mucosa of patients during radiotherapy for head and neck cancer. Radiother Oncol 1991;22:7-11. [Crossref] [PubMed]
- Dörr W. Three A's of repopulation during fractionated irradiation in squamous epithelia: Asymmetry loss, Acceleration of stem-cell divisions and Abortive divisions. Int J Radiat Biol 1997;72:635-43. [Crossref] [PubMed]
- Dische S, Saunders M, Barrett A, et al. A randomised multicentre trial of CHART versus conventional radiotherapy in head and neck cancer. Radiother Oncol 1997;44:123-36. [Crossref] [PubMed]
- Dörr W. Modulation of repopulation processes in oral mucosa: experimental results. Int J Radiat Biol 2003;79:531-7. [Crossref] [PubMed]
- Hopewell JW, Nyman J, Turesson I. Time factor for acute tissue reactions following fractionated irradiation: A balance between repopulation and enhanced radiosensitivity. Int J Radiat Biol 2003;79:513-24. [Crossref] [PubMed]
- Gruber S, Dörr W. Tissue reactions to ionizing radiation-Oral mucosa. Mutat Res 2016;770:292-8. [Crossref] [PubMed]
- Fletcher GH, MacComb WS, Shalek RJ. Radiation therapy in the management of cancer of the oral cavity and oropharynx. Charles Thomas, Springfield IL, 1962.
- Wygoda A, Rutkowski T, Hutnik M, et al. Acute mucosal reactions in patients with head and neck cancer. Three patterns of mucositis observed during radiotherapy. Strahlenther Onkol 2013;189:547-51. [Crossref] [PubMed]
- ICRU. ICRU (International Commission on Radiation Units). Prescribing, recording and reporting photon beam therapy. ICRU Report 62. Oxford: Oxford University Press, 1999.
- Withers HR, Taylor JM, Maciejewski B. Treatment volume and tissue tolerance. Int J Radiat Oncol Biol Phys 1988;14:751-9. [Crossref] [PubMed]
- Dörr W, Van der Kogel AJ. The volume effect in radiotherapy. In: Joiner M, Van der Kogel A. editors. Basic Clinical Radiobiology, 4th ed. London: Hodder Arnold, 2009;191-206.
- Källman P, Agren A, Brahme A. Tumour and normal tissue responses to fractionated non-uniform dose delivery. Int J Radiat Biol 1992;62:249-62. [Crossref] [PubMed]
- Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl 1985;8:S13-9. [Crossref] [PubMed]
- Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: The effective volume method. Int J Radiat Oncol Biol Phys 1989;16:1623-30. [Crossref] [PubMed]
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109-22. [Crossref] [PubMed]
- Marks LB, Ten Haken RK, Martel MK. Guest editor’s introduction to QUANTEC: A users guide. Int J Radiat Oncol Biol Phys 2010;76:S1-2. [Crossref] [PubMed]
- Dörr W. Biological response modifiers: normal tissues. In: Joiner M, Van der Kogel A. editors. Basic Clinical Radiobiology, 4th ed. London: Hodder Arnold, 2009:301-15.
- Horsman MR, Wouters BG, Joiner MC, et al. The oxygen effect and fractionated radiotherapy. In: Joiner M, Van der Kogel A. editors. Basic Clinical Radiobiology, 4th ed. London: Hodder Arnold, 2009:207-16.
- Stone HB, Moulder JE, Coleman CN, et al. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI Workshop, December 3–4, 2003. Radiat Res 2004;162:711-28. [Crossref] [PubMed]
- Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer 2006;6:702-13. [Crossref] [PubMed]
- Jaal J, Richter C, Dörr W. Effect of recombinant human keratinocyte growth factor (Δ23rHuKGF, Palifermin) on inflammatory and immune changes in mouse tongue during fractionated irradiation. Int J Radiat Biol 2010;86:860-6. [Crossref] [PubMed]
- Bjordal JM, Bensadoun RJ, Tunèr J, et al. A systematic review with meta-analysis of the effect of low-level laser therapy (LLLT) in cancer therapy-induced oral mucositis. Support Care Cancer 2011;19:1069-77. [Crossref] [PubMed]
- Carvalho PA, Jaguar GC, Pellizzon AC, et al. Evaluation of low-level laser therapy in the prevention and treatment of radiation-induced mucositis: a double-blind randomized study in head and neck cancer patients. Oral Oncol 2011;47:1176-81. [Crossref] [PubMed]
- Delanian S, Porcher R, Balla-Mekias S, et al. Randomized, placebo controlled trial of combined pentoxifylline and tocopherol for regression of superficial radiation-induced fibrosis. J Clin Oncol 2003;21:2545-50. [Crossref] [PubMed]


