
Expression of Syndecan-2 in gastric adenocarcinoma and its effect on tumorigenesis in vitro
Introduction
Syndecans belong to the family of transmembrane heparan-sulfate proteoglycans, composed of 4 members, Syndecans-1, -2, -3 and -4. Syndecan-2 (Sdc2) is a kind of transmembrane protein with molecular mass of 21 kDa. Its core protein is composed of three domains: the ectodomain containing multiple attachment sites for heparan sulfate side-chains, a single transmembrane domain and a cytoplasmic domain with two highly conserved regions (C1, C2) and a variable region (V) (1).
Sdc2 is mainly expressed by fibroblasts and endothelial cells. Being a co-receptor of growth factor and cytokine (VEGF, TGF-β, interleukin-8 et al.) (2,3), Sdc2 can modulate cell adhesion and migration through certain signaling pathway (4,5). Expressed by microvascular endothelial cells Sdc2 is vital for angiogenesis both in normal and tumor tissues. The shed form of Sdc2 ectodomain can restrain angiogenesis obviously by inhibiting endothelial cell migration in the presence of VEGF. This effect is demonstrated to be mediated by protein tyrosine phosphatase receptor CD148, which leads to a reduction in active β1 integrins on endothelial cells (6). In addition to participation in cell-cell interactions, Sdc2 also mediates cell-extracellular matrix interactions as a cell-surface receptor. During zebrafish embryonic development, Sdc2 which expressed in extra-embryonic tissues regulates fibronectin and laminin matrix assembly, promoting organ primordia migration and fibrillogenesis (7). Although previous studies focused on the roles of Sdc2 in normal cells adhesion and signaling, recently its function of accelerating tumor growth and metastasis has attracted more attention. It has been confirmed to be up regulated in some cancer cells such as breast, pancreatic, colorectal and prostate carcinomas (8-11). Altered expression of Sdc2 can affect migration and invasion of these cancer cells. Lim et al. recently found that depletion of Sdc2 by siRNA could reduce invasion activity of breast carcinoma cell line MDA-MB231 by regulating the RhoGTPases (8). Vicente et al. demonstrated that Sdc2 expression was enhanced by stromal fibroblasts in highly metastatic colorectal cancer cell line, HCT-116. A decrease in HCT-116 cell adhesion, migration, and organization of actin filaments was due to the blocking Sdc2 by a specific antibody (10).
It is clear that Sdc2 can affect the behaviors of some cancer cells. However it is poorly understood in terms of its role in tumorigenesis of gastric carcinoma. Here we conducted retrospective studies on patients with gastric adenocarcinoma and attempted to determine the expression and prognostic role of Sdc2. Besides, in vitro experiments clarified how Sdc2 affected the biological behaviors of gastric adenocarcinoma cells.
Methods
Patients and tissue sampling
The specimens of gastric adenocarcinoma used in this study were obtained from surgical procedures. Forty-six patients underwent radical gastrectomy for gastric adenocarcinoma at Department of Surgery, Huadong Hospital affiliated to Fudan University, between 2010 and 2013. None of the patients was treated with neoadjuvant therapy. The age range was 32–82 years (average: 64 years), including 32 men and 14 women. One case of tumor was located at pyloric, 7 cases at cardia, 13 cases at gastric antrum, 20 cases at gastric body and 5 cases at gastric fundus. The tumor-node-metastasis (TNM) classification was assigned by the pathologist according to the criteria of the American Joint Committee on Cancer (7th ed, 2010) (12). Two (4.35%) patients had stage T0, 1 (2.17%) stage T1, 3 (6.52%) stage T2, 6 (13.04%) stage T3 and 34 (73.92%) had stage T4 disease. Thirty-two (69.57%) patients had metastases in regional lymph nodes. No one had distant metastases. The adjacent normal tissues were used as control. Median follow-up was 31 months (IQR, 21.1–45.6). Disease-free survival (DFS, defined as the time from curative treatment to the date of disease recurrence or death due to disease progression). Overall survival (OS, defined as the time from curative treatment to the death from any cause).
Immunohistochemistry staining and evaluation
Specimens were fixed in 4% buffered formalin, embedded in paraffin, cut at 2.5 µm thickness. Sections were deparaffinized, alcohol hydrated, heat-induced epitope retrieved and endogenous peroxidase inactivated in sequence. Then primary antibody for Sdc2 (dilution 1:50; Life Span BioSciences, Inc., USA) was added to the sections. Afterwards sections were incubated for 2 hours at room temperature. After washing in PBS for three times, sections were incubated with EnVision kit (DAKO Corp., Denmark) for 30 minutes at 37 °C. Stained with diaminobenzidine (DAKO Corp., Denmark), sections were counterstained with Mayer’s hematoxylin and mounted. For a negative control, staining was performed without the primary antibody. Results of slides were assessed by two pathological doctors in a double-blind method. Staining intensity was scored as 0 (negative), 1 (weak), 2 (moderate) and 3 (strong). The percentage of positive cells were divided into 0 (no positive cell), 1 (≤25%), 2 (26–50%), 3 (51–75%), 4 (>75%). According to the product of staining intensity and the percentage of positive cells, the immunohistochemical result was classified as negative-low expression (<4) and moderate-strong expression (≥4).
Cell cultures
Human gastric carcinoma cell lines MKN-45, MGC-803, BGC-823 and SGC-7901 were obtained from Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China). All cells were maintained in RPMI1640 (Gibco BRL Co.Ltd., USA), supplemented with 10% fetal bovine serum (Tianhang Biotechnology Co.Ltd., China) at 37 °C in a humidified atmosphere of 5% CO2 in air.
Western blot analysis
To detect Sdc2 protein, western blot analyses were performed following SDS-PAGE. Protein samples (50 µg) were separated by SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Merck Millipore, Germany). The membranes were blocked with 5% non-fat milk for 2 hours at room temperature, then immunoblotted with primary antibody (Life Span BioSciences, Inc., USA) and secondary antibody (Proteintech Group, Inc., USA) successively. Specific binding was detected with enhanced chemiluminescence system (Tanon 5200S, Tanon Science & Technology Co. Ltd., China).
Real-time PCR
Total RNA was extracted from gastric carcinoma cells using a TRIzol kit (Invitrogen, USA). Total RNA (2 µg) was reverse transcribed to cDNA using TransScript® One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech, China) and then amplified using following primers: Sdc2, 5'-AAACGGACAGAAGTCCTAGC-3' and 5'-GATAAGCAGCACTGGATGGT-3' (GAPDH was used as internal control). The amplification reactions were performed using a DNA Thermal Cycler (Bio-Rad, USA) with 39 cycles of sequential denaturation (95 °C for 15 seconds), annealing (58 °C for 30 seconds) and extension (65 °C for 5 minutes). DNA fragments amplified by PCR were separated by electrophoresis on 1.5% agarose gels containing ethidium bromide.
Cell transfection
We designed three pairs of double-strand siRNA oligonucleotide targeting Sdc2 (siRNA-1, sense 5'-GAAACCACGACGCUGAAUAdTdT-3', antisense 5'-UAUUCAGCGUCGUGGUUUCdTdT-3'; siRNA-2, sense 5'-GUUGGUGUAUCGCAUGAGAdTdT-3', antisense 5'-UCUCAUGCGAUACACCAACdTdT-3'; siRNA-3, sense 5'-GUAUCCUAUUGAUGACGAUdTdT-3'; antisense 5'-AUCGUCAUCAAUAGGAUACdTdT-3') synthesized by BGI (Beijing Genomics Institution, China). 24 hours before transiently transfected with Sdc2-siRNA or non-targeting control siRNA, cells (1×105 per well) were seeded into 6-well plates. Sdc2-siRNA of 5 µL and DNA transfection reagent of 10 µL were premixed in OPTI medium (Invitrogen Life Technologies, CA) for 20 minutes and then applied to the cells (500 µL per well). Cells were incubated at 37 °C in a humidified atmosphere of 5% CO2, after transfection for 6 hours, OPTI medium was replaced with fresh culture medium.
Cell migration and invasion assays
The migration assays were performed using Costar transwell inserts (pore size, 8 µm; Corning, NY, USA) in 24-well plates. Before the migration assay, cells were cultured in a serum-free medium for 12 hours. Cells (5×104) were seeded into each upper chamber and 600 µL of serum medium was placed in the lower chamber. Separately after being incubated at 37 °C in 5% CO2 for 24 and 48 hours, the cells that migrated through the filter were fixed in methanol for 15 minutes and stained with 0.2% crystal violet. The cell number was counted under a microscope at 100× magnification and showed as the fold of control. For the invasion assays, 24-well transwell plates (pore size, 8 µm; Corning, NY, USA) were coated with gelatin (10 µg/mL) on the lower side of the membrane and with matrigel(30 µg/mL; BD, USA) at a thickness of 5 mm on the upper side. Each experiment was repeated at least three times.
Statistical analysis
Statistical analyses were performed using SPSS for Windows, version 16.0 (SPSS Inc., USA). Counting data between groups were analyzed by using Chi-square test. Survival rates were visualized applying the Kaplan-Meier curves and log rank test. Cell migration and invasion assays were analyzed by using two-sample Student t-test. P values less than 0.05 were considered significant.
Results
Sdc2 is expressed in gastric adenocarcinoma
To explore the role of Sdc2 in the development of gastric adenocarcinoma, the expression level of Sdc2 was compared in neoplastic tissues and normal tissues. In normal gastric tissues, moderate-to-strong staining for Sdc2 was observed in endothelial cells and fibroblasts, but negative in epithelial cells (Figure 1A). On the contrary, Sdc2 expression was obviously increased in neoplastic tissues compared with normal tissues (Figure 1B,C).
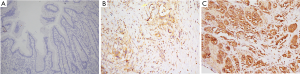
The high expression of Sdc2 in tumor tissues is correlated with some aggressive pathological features
To explore the function of Sdc2 in tumor tissues, we analyzed the association between the expression of Sdc2 and clinicopathologic characteristics. According to expression intensity (refer to immunohistochemical staining and evaluation) 14 (30.43%) sections with negative-low expression (<4) were designated as Group A, and 32 (69.57%) sections with moderate-strong expression (≥4) were designated as Group B. Immunohistochemical reaction was cytoplasmatic. As shown in Table 1, rates of local deep invasion (P=0.03), neural invasion (P=0.035) and lymph node metastasis (P=0.009) were significantly higher in Group B than those were shown in Group A. There was no correlation between Sdc2 expression, tumor size (P=0.246), tumor differentiation (P=0.2) and tumor thrombosis (P=0.559). These results suggested that the expression of Sdc2 was associated with aggressive biological behaviors of gastric adenocarcinoma.
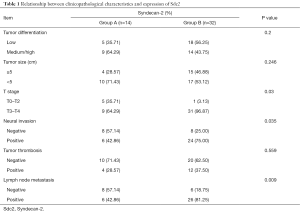
Full table
Overexpression of Sdc2 in tumor tissues leads to shortened survival
To investigate whether the expression intensity of Sdc2 was correlated with survival period, we followed up these 46 patients and analyzed survival time. Median DFS and OS in Group B was 19.0 months (95% CI, 5.9–32.1) and 28.7 months (95% CI, 17.1–40.3) respectively, but neither had reached in Group A. Analysis of survival showed that patients with moderate-strong expression of Sdc2 had a trend toward decreased DFS (P=0.142; Figure 2A) and OS (P=0.068; Figure 2B). There was no significant differences in rates of 2-year DFS (71.43% vs. 46.88%; P=0.224) and OS (85.71% vs. 53.13%; P=0.076) between two groups. Accordingly, the trends of shortened survival and low survival rate indicated that up-regulation of Scd2 expression might predict adverse clinical outcome.
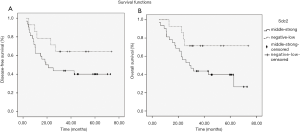
Expression levels of Sdc2 in different gastric carcinoma cell lines
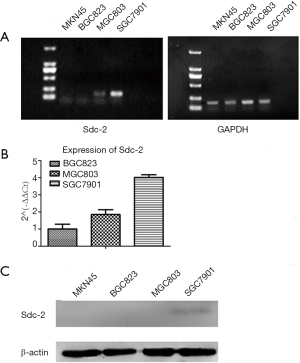
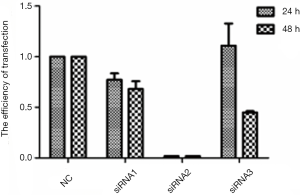
In order to detect the expression levels of Sdc2 in different gastric carcinoma cell lines, RT-PCR experiments were carried out and revealed that the human gastric carcinoma cell lines BGC-823, MGC-803 and SGC-7901 expressed Sdc2 mRNA (Figure 3A,B) except MKN-45 cells. Western-blot analysis was performed to detect expression of Sdc2 protein (Figure 3C) and proved that it could be detected only in SGC-7901 cells. Based on the above results, SGC-7901 cells were elected for the following experiment. Afterwards, we proved that Sdc2 expression in SGC-7901 cells was significantly decreased using Sdc2 siRNA (Figure 4).
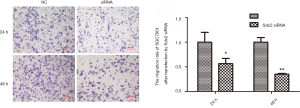
Sdc2 suppression inhibits cell migration and invasion in vitro
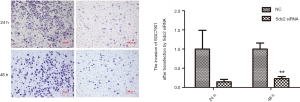
To determine the biological role of Sdc2 expression in migration/invasion of gastric carcinoma cells, we examined the migration and invasion activity of SGC-7901 cells using transwell assays. Compared with cells transfected with non-targeting control siRNA, migration ability of SGC-7901 cells which were transfected with Sdc2-siRNA was decreased obviously (Figure 5). Cell invasion ability was also significantly restrained after Sdc2 expression was suppressed by siRNA (Figure 6). These data suggested that Sdc2 could regulate cell migration and invasion in gastric carcinoma cells.
Discussion
Increasing number of findings have confirmed that Sdc2 plays an important role in the development and progression of tumor. The expression level of Sdc2 is up-regulated in certain tumor tissues. In our study, Sdc2 expression and relationship with clinicopathological characteristics and disease prognosis were assessed in a cohort of 46 patients treated with radical gastrectomy for gastric adenocarcinoma. Immunohistochemistry results demonstrated that in addition to endothelial cells and fibroblasts, Sdc2 was also found in the majority of tumor cells, but negative in epithelial cells of normal gastric tissues. Further in vitro experiments had shown that the human gastric carcinoma cell line SGC-7901 expressed both Sdc2 mRNA and protein. The above results were consistent with previous findings where Sdc2 expression was enhanced in colorectal cancer tissues both in vivo and in vitro (13,14). Therefore, we speculate that Sdc2 up-regulated expression is a common phenomenon of gastrointestinal malignancy.
Furthermore, correlation was found between the expression of Sdc2 in tumor tissues and disease prognosis. In Popovic’s study (11), 86 prostate cancer patients treated with radical prostatectomy were assessed. Immunohistochemical staining results showed that normal prostatic epithelial tissue and stroma did not express Sdc2. To the contrary, Sdc2 expression was present in the majority of prostate cancers (76.1%). There was a notable correlation between Sdc2 overexpression in prostate cancer and established features indicative of worse prognosis. Similar findings were reported in the study of colorectal cancer that overexpression of Sdc2 was associated with pathological features of poor prognosis (13). Our results on Sdc2 expression in gastric adenocarcinoma were consistent with these earlier reports. A moderate-strong Sdc2 expression in tumor lesion was associated with high probability of local deep infiltration, neural invasion, and lymph node metastasis. Although there was no significant difference in survival analysis between two groups, partially due to small sample size and the interference of different adjuvant therapy regimens, we found patients with moderate-strong expression of Sdc2 had a trend toward decreased DFS and OS. Therefore, our results supported the potential role of Sdc2 as a prognostic factor.
How does Sdc2 promote the process of tumor growth and progression? There are two main mechanisms according to the results of previous researches. First, Sdc2 was able to strengthen motility and increase invasion ability of cancer cells. Under physiological conditions, its cytoplasmic domain C1 could interact with proteins of the ERM family (ezrin, radixin, and moesin) (15). These proteins were one of the key molecules which linked the actin cytoskeleton to the plasma membrane and were implicated in cells including cancer cells morphogenesis, migration and adhesion (16,17). During cancer progression, Sdc2 promoted migration and metastasis through certain cellular signal transduction pathways. In study of breast carcinoma cell line MDA-MB231, it was proved that depletion of Sdc2 gave rise to the decline of invasiveness, reducing cell migration accompanied by translocation of p190ARhoGAP to the cell edge and its increased tyrosine phosphorylation (8). TGFβ2/Smad2 was another signal pathway that got involved in. By using its transmembrane heparan sulfate chains, Sdc2 interacted with members of the TGFβ superfamily (7). It was demonstrated that there was a positive feedback mechanism between Sdc2 expression and TGFβ signaling in fibrosarcoma cells. In Sdc2-deficient fibrosarcoma cells, TGFβ2-dependent up-regulation of Smad2 phosphorylation level was suppressed, subsequently its downstream transcriptional regulation of integrin β1 was dramatically inhibited leading to the alteration of adhesion (18). In our study, down-regulation of Sdc2 inhibited SGC-7901 cell migration and invasion. Agreed with previous studies, we proved that Sdc2 had a strong effect on maintaining an invasive phenotype of gastric carcinoma cells.
In addition to its roles in cell adhesion and migration, Sdc2 is necessary for tumor angiogenesis that promotes tumor growth and metastasis. Several studies have shown that Sdc2 could physically interact with cell-cell signaling molecules that stimulated angiogenesis (e.g., VEGF, bFGF) (2,19). These pro-angiogenic growth factors have been illustrated to induce Sdc2 shedding from brain microvascular endothelial cells and then facilitate angiogenesis of gliomas in vitro (20).
There are abundant evidences to demonstrate that Sdc2 can promote the development and progression of certain malignant tumors, but the mechanism is not completely clear. The results presented in this study suggest that Sdc2 plays a crucial role in the migration and invasion of gastric carcinoma cells. The expression of Sdc2 in gastric adenocarcinoma tissues is up-regulated and has the potential to be a prognostic factor for patients with gastric cancer. Further investigations will be required to clarify the mechanism how Sdc2 stimulates tumorigenesis and metastasis in gastric cancer.
Acknowledgments
The authors are grateful to Prof. Jian-Wen Liu and Ms Cen Qiu (State Key Laboratory of Bioreactor Engineering & Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, Shanghai, China) for instruction, and grateful to Dr. Qi Lu (Department of Surgery, Huadong Hospital Affiliated to Fudan University, Shanghai, China) for providing specimens of gastric adenocarcinoma.
Funding: This study was funded by Shanghai Municipal Commission of Health and Family Planning, “Scientific research project” (No. 20164Y0134) and Shanghai Municipal Commission of Health and Family Planning, Key developing disciplines (No. 2015ZB0501).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.08.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Institutional ethical approval was waived. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Essner JJ, Chen E, Ekker SC. Syndecan-2. Int J Biochem Cell Biol 2006;38:152-6. [Crossref] [PubMed]
- Chen E, Hermanson S, Ekker SC. Syndecan-2 is essential for angiogenic sprouting during zebrafish development. Blood 2004;103:1710-9. [Crossref] [PubMed]
- Halden Y, Rek A, Atzenhofer W, et al. Interleukin-8 binds to syndecan-2 on human endothelial cells. Biochem J 2004;377:533-8. [Crossref] [PubMed]
- Kramer KL, Yost HJ. Heparan sulfate core proteins in cell-cell signaling. Annu Rev Genet 2003;37:461-84. [Crossref] [PubMed]
- Tkachenko E, Rhodes JM, Simons M. Syndecans: new kids on the signaling block. Circ Res 2005;96:488-500. [Crossref] [PubMed]
- De Rossi G, Evans AR, Kay E, et al. Shed syndecan-2 inhibits angiogenesis. J Cell Sci 2014;127:4788-99. [Crossref] [PubMed]
- Arrington CB, Yost HJ. Extra-embryonic syndecan 2 regulates organ primordia migration and fibrillogenesis throughout the zebrafish embryo. Development 2009;136:3143-52. [Crossref] [PubMed]
- Lim HC, Couchman JR. Syndecan-2 regulation of morphology in breast carcinoma cells is dependent on RhoGTPases. Biochim Biophys Acta 2014;1840:2482-90. [Crossref] [PubMed]
- Hrabar D, Aralica G, Gomercic M, et al. Epithelial and stromal expression of syndecan-2 in pancreatic carcinoma. Anticancer Res 2010;30:2749-53. [PubMed]
- Vicente CM, Ricci R, Nader HB, et al. Syndecan-2 is upregulated in colorectal cancer cells through interactions with extracellular matrix produced by stromal fibroblasts. BMC Cell Biol 2013;14:25. [Crossref] [PubMed]
- Popović A, Demirovic A, Spajic B, et al. Expression and prognostic role of syndecan-2 in prostate cancer. Prostate Cancer Prostatic Dis 2010;13:78-82. [Crossref] [PubMed]
- Edge SB, Byrd DR, Compton CC. AJCC Cancer Staging Handbook. 7th ed. New York: Springer-Verlag, 2010.
- Wei JL, Fu ZX, Fang M, et al. High expression of CASK correlates with progression and poor prognosis of colorectal cancer. Tumour Biol 2014;35:9185-94. [Crossref] [PubMed]
- Jang B, Jung H, Chung H, et al. Syndecan-2 enhances E-cadherin shedding and fibroblast-like morphological changes by inducing MMP-7 expression in colon cancer cells. Biochem Biophys Res Commun 2016;477:47-53. [Crossref] [PubMed]
- Granés F, Urena JM, Rocamora N, et al. Ezrin links syndecan-2 to the cytoskeleton. J Cell Sci 2000;113:1267-76. [PubMed]
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 2002;3:586-99. [Crossref] [PubMed]
- Clucas J, Valderrama F. ERM proteins in cancer progression. J Cell Sci 2015;128:1253. [Crossref] [PubMed]
- Mytilinaiou M, Bano A, Nikitovic D, et al. Syndecan-2 is a key regulator of transforming growth factor beta 2/Smad2- mediated adhesion in fibrosarcoma cells. IUBMB Life 2013;65:134-43. [Crossref] [PubMed]
- Alexopoulou AN, Multhaupt HA, Couchman JR. Syndecans in wound healing, inflammation and vascular biology. Int J Biochem Cell Biol 2007;39:505-28. [Crossref] [PubMed]
- Fears CY, Gladson CL, Woods A. Syndecan-2 is expressed in the microvasculature of gliomas and regulates angiogenic processes in microvascular endothelial cells. J Biol Chem 2006;281:14533-6. [Crossref] [PubMed]


