
Brigatinib entering the clinic for ALK rearranged metastatic NSCLC: editorial on a randomized multicenter phase II study with two brigatinib dose regimens
Introduction
In ~5% of patients with non-small-cell lung cancer (NSCLC), the disease is characterized by an anaplastic lymphoma kinase (ALK) rearrangement (1,2). In 2011 the Food and Drug Administration granted Crizotinib, an ALK tyrosine kinase inhibitor (TKI), accelerated approval based on durable objective response rates (ORR) of 50 percent and 61 percent in two single-arm open-label studies (3-5). A signal that was confirmed in a randomized phase III study where crizotinib showed superior outcome when compared to pemetrexed or docetaxel monotherapy in patients that progressed after platinum doublet chemotherapy (6).
Crizotinib moved to the first line based on a randomized phase III study that showed superior outcome with first line crizotinib treatment as compared with platinum-doublet chemotherapy (7). Crizotinib resulted in an ORR of 74% (vs. 45% with chemotherapy) and a progression-free survival (PFS) of 10.9 months (vs. 7.0 months with chemotherapy). Since then, the field of ALK TKI development moved quickly. Two ALK TKIs received approval by the FDA for the treatment of patients with ALK-rearrangement positive NSCLC that progressed on crizotinib. Ceritinib showed superior outcome when compared to pemetrexed or docetaxel monotherapy in patients that progressed after at least one line of chemotherapy and crizotinib (8). The majority of patients (82%) received crizotinib at the time of study enrollment. Ceritinib resulted in an ORR of 45% and a PFS of 5.4 months. Alectinib showed efficacy in two single arm studies in patients that progressed while receiving crizotinib (9,10). The majority of patients (80% and 74%) received one of more lines of prior chemotherapy. The ORR was 50% and 48% and the PFS 8.9 months and 8.1 months. Since then, both drugs received FDA approval for first line treatment as well. Ceritinib showed superior outcome when compared to platinum-doublet chemotherapy (11). Ceritinib resulted in an ORR of 73% (vs. 27% with chemotherapy) and a PFS of 16.6 months (vs. 8.1 months with chemotherapy). Alectinib showed superior outcome when compared with crizotinib (12). Alectinib resulted in an ORR of 83% (vs. 76% with crizotinib) and the median PFS was not yet reached with alectinib (95% CI: 17.7 months–not yet reached) vs. 11.1 months with crizotinib.
Central nervous system (CNS)
ALK positive NSCLC has a high probability to metastasize to the CNS. Up to 60% of patients develop CNS metastases during the course of their disease (6,13) and ~20% of patients present with CNS metastases at the time of diagnosis (6,14). With crizotinib treatment the CNS is a preferential site for progression of disease. In the crizotinib registration studies, 70% of the patients with brain metastases prior to crizotinib initiation, had a new lesions or non-target progression in the CNS at the time of disease progression, while this was 20% for patients without CNS metastases at presentation (13). Besides drug resistance mechanisms, the limited CNS penetration of crizotinib is likely to play a role (15). Ceritinib and alectinib both demonstrated to be active in controlling and treating brain metastases, both in crizotinib naïve patients (11,12) and patients that progressed in the CNS after crizotinib failure (8-10). In patients that failed crizotinib and had active CNS metastases at study enrollment, ceritinib resulted in ORRs of 35% and 45% in the phase III and II trials, respectively (8,16). Alectinib resulted in ORRs of 57% and 75% in two single arm phase II studies (9,10).
Brigatinib in patients with crizotinib-refractory ALK-positive non-small-cell lung cancer: a randomized, multicenter phase II trial
In a randomized phase II trial Kim et al. evaluated two dose regimens of brigatinib, a second generation ALK TKI, in patients with ALK-rearrangement positive NSCLC that progressed on crizotinib at the time of study enrollment (17). Any number of prior chemotherapy regimens was allowed. The patients (n=222) were 1:1 randomized to oral brigatinib 90 mg once daily (arm A) or 180 mg once daily with a 7-day lead-in of 90 mg once daily (arm B) because of pulmonary toxicity that was encountered in the phase I/II trial with a starting dose of 180 mg (18). Patients were stratified by baseline brain metastases (present vs. absent) and best investigator-assessed response to crizotinib (response vs. other or unknown). Treatment was allowed to be continued at the investigator’s discretion after progression. A contrast-enhanced MRI of the brain was required at screening and follow-up imaging was done every 8 weeks. The primary end-point was confirmed ORR per RECIST v1.1 (per investigator). Secondary end points included confirmed ORR [per central independent review committee (IRC)], CNS response, duration of response, PFS, overall survival (OS), safety, tolerability, and quality-of-life. A sample size of ≥109 patients in each arm was calculated to provide 90% power to rule out an ORR of 20% when the true ORR would be ≥35% with a two-sided alpha level of 0.025. The trial was not designed for statistical comparisons between the two dosing arms. 112 patients were allocated to arm A (90 mg arm) and 110 to arm B (180 mg). In arms A and B 71% and 67% had brain metastases at the time of study enrollment, respectively, and 74% received prior chemotherapy in both arms. Investigator-assessed confirmed ORR was 45% (97.5% CI, 34% to 56%) in arm A and 54% (97.5% CI, 43% to 65%) in arm B. Investigator-assessed median PFS was 9.2 months (95% CI, 7.4–15.6) and 12.9 months (11.1–not yet reached) in arms A and B, respectively. IRC-assessed intracranial ORR in patients with measurable baseline brain metastases was 42% (11 of 26 patients; 95% CI, 23% to 63%) in arm A and 67% (12 of 18 patients; 95% CI, 41% to 87%) in arm B.
The most common treatment-emergent adverse events (AEs) in arms A and B were nausea (33%/40%), diarrhea (19%/38%), headache (28%/27%) and cough (18%/34%). The most common grade ≥3 AEs were hypertension (6%/6%), increased blood creatine phosphokinase (3%/9%), pneumonitis (3%/5%) and increased lipase (4%/3%). Early onset pulmonary AEs [median time to onset, 2 days (range, 1 to 9 days)] occurred in 14 patients (6%) and included dyspnea, hypoxia, cough, pneumonia, or pneumonitis. These AEs occurred at 90 mg in both arms and no such events occurred after escalation to 180 mg. In seven patients (3%) this event was ≥ grade 3. They were managed with dose interruption and successful reintroduction of brigatinib was possible in 6 of 14 patients. One patient continued treatment with resolution of symptoms after dose reduction to 60 mg once daily without needing dose interruption. Seven patients discontinued treatment, including one patient who died on day 7, after experiencing dyspnea, cough, and pneumonia. This patient’s autopsy revealed malignant pleural effusion, widespread lung scarring, and diffuse alveolar damage.
Dose reduction as the result of any AE occurred in 7% and 20% of treated patients in arms A and B, respectively. Dose interruption (≥3 days) for any reason occurred in 18% and 36% of patients in arms A and B, respectively. The most common reasons for dose reduction were increased blood creatine phosphokinase, pneumonitis, and rash.
Based on these results, the FDA granted brigatinib accelerated approval as a treatment for patients with ALK-rearranged NSCLC who are resistant to prior crizotinib.
Sequencing of ALK TKIs and the position of chemotherapy
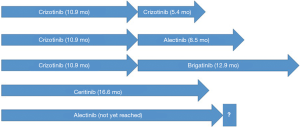
With three drugs (alectinib, ceritinib and crizotinib) having FDA approval for the treatment of ALK TKI naïve ALK-rearranged metastatic NSCLC and three drugs having a label for the second line setting after crizotinib failure (alectinib, brigatinib and ceritinib), it is unclear what the best strategy is to sequence the available ALK TKIs. In this trial brigatinib showed an ORR that is similar to that of alectinib and ceritinib in patients that progressed on crizotinib. Although the trial was not powered to compare the two dosing arms, efficacy outcomes favored the higher dose, most notably in PFS and intracranial response. At a dose of 180 mg, the intracranial ORR was 67%. Although formal comparisons are not available this seems to be equivocal to what can be seen with alectinib (9,10). The median PFS of 9.2 and 12.9 months compare favorably to that of what can be obtained with ceritinib or alectinib after crizotinib failure, although again, formal comparisons are not available (Figure 1). It remains an open question what strategy leads to the longest OS; start with a second generation ALK TKI or start with crizotinib and a second generation ALK TKI upon crizotinib failure, of which brigatinib may be the winner, based on PFS. The best way to answer this question is by performing head-to-head studies with a cross-over design. Unfortunately the recent ALEX study with first line alectinib vs. crizotinib did not allow for cross-over and therefore it remains unanswered what strategy results in the longest (combined) PFS (first line alectinib or sequential crizotinib and alectinib). The ongoing ALTA-1L study with first line brigatinib vs. crizotinib does allow for cross-over to brigatinib in patients that are randomized to crizotinib and hopefully this will answer the sequencing question of crizotinib and brigatinib (19).
Another open question is the efficacy of second generation ALK TKIs after progression on treatment with another second generation ALK TKI. Especially now that two second generation ALK TKIs (alectinib and ceritinib) received FDA approval for first line treatment of ALK-rearranged NSCLC. The emergence of ALK mutations is more common after second generation ALK TKI treatment than crizotinib and the individual ALK TKIs show different ALK mutation profiles at the time of disease progression, possibly resulting in cross-sensitivity (20). A publication by Shaw et al. showed that monitoring ALK mutation status can guide sequencing of ALK TKIs in a case where the emergence of a L1198F mutation resensitized ALK-rearranged NSCLC to crizotinib after lorlatinib failure, a next generation ALK TKI (21). Analogous to the EGFR setting, mutation testing both in plasma and tissue enables to monitor resistance and might guide treatment.
Adding to the complexity, (platinum doublet) chemotherapy remains a treatment option with clinical efficacy with an ORR of 27–45% and a PFS of 7.0–8.1 months (7,11). As an ‘off-ALK’ treatment it targets ALK-rearranged NSCLC through a different mechanism and offers an ALK TKI drug holiday and might sensitize the tumor to retreatment with ALK TKIs (22).
Conclusions
Brigatinib showed an excellent response rate in patients with ALK-rearrangement positive NSCLC that failed crizotinib. Once daily 180 mg with a 7-day lead-in of 90 mg once daily is the preferred dose with a high overall and CNS response rate and a manageable toxicity profile. PFS compares favorably with that of alectinib and ceritinib after crizotinib failure. Questions that remain unanswered are the best way to sequence ALK TKIs (first line crizotinib followed by a second generation ALK TKI or first line treatment with a second generation ALK TKI) and the efficacy of second generation ALK TKIs after progression on another second generation ALK TKI.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Shaohua Cui (Department of Pulmonary Medicine, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China).
Conflicts of Interest: The author attended advisory boards of AstraZeneca, Boehringer, BMS, Lilly, MSD and Pfizer and received research grants from AstraZeneca, BMS and MSD.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pikor LA, Ramnarine VR, Lam S, et al. Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung Cancer 2013;82:179-89. [Crossref] [PubMed]
- Sholl LM, Aisner DL, Varella-Garcia M, et al. Multi-institutional Oncogenic Driver Mutation Analysis in Lung Adenocarcinoma: The Lung Cancer Mutation Consortium Experience. J Thorac Oncol 2015;10:768-77. [Crossref] [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [Crossref] [PubMed]
- Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 2012;13:1011-9. [Crossref] [PubMed]
- Kim DW, Ahn MJ, Shi Y, et al. Results of a global phase II study with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC). J Clin Oncol 2012;30:abstr 7533.
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Shaw AT, Kim TM, Crino L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2017;18:874-86. [Crossref] [PubMed]
- Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 2016;17:234-42. [Crossref] [PubMed]
- Ou SH, Ahn JS, De Petris L, et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 2016;34:661-8. [Crossref] [PubMed]
- Soria JC, Tan DS, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Costa DB, Shaw AT, Ou SH, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol 2015;33:1881-8. [Crossref] [PubMed]
- Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015;88:108-11. [Crossref] [PubMed]
- Crinò DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 2011;29:e443-5. [Crossref] [PubMed]
- Crino L, Ahn MJ, De Marinis F, et al. Multicenter Phase II Study of Whole-Body and Intracranial Activity With Ceritinib in Patients With ALK-Rearranged Non-Small-Cell Lung Cancer Previously Treated With Chemotherapy and Crizotinib: Results From ASCEND-2. J Clin Oncol 2016;34:2866-73. [Crossref] [PubMed]
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol 2017;35:2490-8. [Crossref] [PubMed]
- Gettinger SN, Bazhenova LA, Langer CJ, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol 2016;17:1683-96. [Crossref] [PubMed]
- Tiseo M, Popat S, Gettinger SN, et al. Design of ALTA-1L (ALK in lung cancer trial of brigatinib in first-line), a randomized phase 3 trial of brigatinib (BRG) versus crizotinib (CRZ) in tyrosine kinase inhibitor (TKI)-naive patients (pts) with advanced anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC). J Clin Oncol 2017;35:abstr TPS9098.
- Gainor JF, Dardaei L, Yoda S, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- Shaw AT, Friboulet L, Leshchiner I, et al. Resensitization to Crizotinib by the Lorlatinib ALK Resistance Mutation L1198F. N Engl J Med 2016;374:54-61. [Crossref] [PubMed]
- Matsuoka H, Kurata T, Okamoto I, et al. Clinical response to crizotinib retreatment after acquisition of drug resistance. J Clin Oncol 2013;31:e322-3. [Crossref] [PubMed]


