
The importance of RET-directed therapy in patients with RET-rearranged non-small cell lung cancer
Non-small cell lung cancer (NSCLC) has been recognized as a heterogeneous set of diseases according to oncogenic gene alterations level, such as sensitizing EGFR mutations, ALK rearrangements, or ROS1 rearrangements (1). Specific molecularly targeted therapies are effective in NSCLC patients harboring sensitizing EGFR mutations, ALK or ROS1 rearrangements, with response rates of approximately 60% and median progression-free survival of over 10 months (2-4). RET is a proto-oncogene that codes for a transmembrane protein belonging to the receptor tyrosine kinases family (5). In 2012, RET rearrangements were identified as new oncogenic alterations occurring in 1% to 2% of patients with NSCLC (6-9). It is reported that RET rearrangements has tumor-driving activity in vitro and in vivo. RET can partner with different genes in NSCLC, and KIF5B is the most common fusion partner in NSCLC. To date, several other genes including CCDC6, NCOA, TRIM33, CUX1, KIAA1468, KIAA1217, and FRMD4A, have been identified as other fusion partners of RET in NSCLC patients. RET rearrangements tend to be found in younger patients, female, never or former light smokers, and in patients with lung adenocarcinomas (10). A few case reports reported that cabozantinib and vandetanib, which are multi-targeted tyrosine kinase inhibitor exhibiting RET kinase activity, have antitumor activity in patients with RET-rearranged NSCLC (11-13).
The Global, Multicenter RET Registry (GLORY) is the largest single database of patients with RET-rearranged NSCLC. In a recent paper by Gautschi et al. (14), GLORY presented the results of independent retrospective and prospective series that described clinicopathologic features of RET-rearranged NSCLC and collected real-world data on the use of RET-directed, targeted therapy outside of clinical protocols. From June 2015 to April 2016, 165 patients with RET-rearranged NSCLC from 29 centers in 12 countries across Europe, Asia, and the United States were accrued in GLORY. Median age was 61 years (range, 29 to 89 years) and the percentage of males and females was balanced. The majority of patients were never smokers (63%) and the predominant histology was lung adenocarcinoma (98%). Most patients (72%) had stage IV disease at diagnosis. Molecular testing for RET was performed locally via fluorescence in situ hybridization (FISH), next-generation sequencing (NGS), and real-time polymerase chain reaction (RT-PCR). The fusion partners were identified in 81 (49%) of 165 patients. KIF5B was the most common partner and was found in 58 patients (72%), followed by CCDC6 in 19 patients (23%), NCOA4 in two patients (2%), EPHA5 in one patient (1%), and PICALM in one patient (1%).
Fifty-three (33%) of 162 patients with RET-rearranged NSCLC received a RET inhibitor during the course of therapy. RET inhibitors included cabozantinib in 21 patients, vandetanib in 11 patients, sunitinib in 10 patients, sorafenib in two patients, alectinib in two patients, lenvatinib in two patients, nintedanib in two patients, ponatinib in two patients, and regorafenib in one patient. Among the 50 assessable patients by RECIST version 1.1, the best response was complete response in 2 patients (4%), partial response in 11 patients (22%), stable disease in 16 patients (32%), progressive disease in 20 patients (40%), and not evaluable in 1 patient (2%). Responses were observed with cabozantinib, vandetanib, sunitinib, lenvatinib, and nintedanib, but not with sorafenib, alectinib, ponatinib, or regorafenib. Median progression-free survival (PFS) was 2.3 months (95% CI, 1.6 to 5.0 months). Median overall survival (OS) was 6.8 months (95% CI, 3.9 to 14.3 months). Analysis of the efficacy according to RET inhibitors was performed. In 19 patients who were treated with cabozantinib, the response rate was 37% and median PFS was 3.6 months. In 11 patients who were treated with vandetanib, the response rate was 18% and median PFS was 2.9 months. In 9 patients who were treated with sunitinib, the response rate was 22% and median PFS was 2.2 months. GLORY indicated that available multi-targeted tyrosine kinase inhibitors had limited activity of patients with RET-rearranged NSCLC.
Several phase 2 trials were performed to investigate the activity of single-agent RET inhibitors in patients with RET-rearranged NSCLC. The summary is listed in Table 1. Drilon et al. (15) reported the results of a phase II trial to evaluate the activity of cabozantinib in 26 patients with RET-rearranged NSCLC. The objective response rate (ORR) in the 25 assessable patients was 28% [95% CI (12%, 49%)]. The median PFS was 5.5 months [95% CI (3.8, 8.4)], and the median OS was 9.9 months [95% CI (8.1, not reached)]. We reported the results of a phase II trial to evaluate the activity of vandetanib in 19 patients with RET-rearranged NSCLC (16). Among 17 eligible patients included in primary analysis, the ORR was 53% [95% CI (28%, 77%)]. The median PFS was 4.7 months [95% CI (2.8, 8.5)], and the median OS was 11.1 months [95% CI (9.4, not reached)]. Lee et al. (17) reported the results of a phase II trial to evaluate the activity of vandetanib in 18 Korean patients with RET-rearranged NSCLC. Among 17 eligible patients, the ORR was 18%, the median PFS was 4.5 months, and the median OS was 11.6 months.
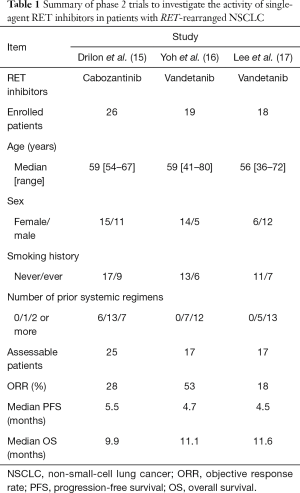
Full table
The efficacy of single-agent RET inhibitors in these clinical trials was more promising than the response rates of 10% to 20% reported for second-line therapy in molecularly unselected NSCLC patients. However, this efficacy was lower than that of targeted therapy in NSCLC patients harboring sensitizing EGFR mutations, ALK or ROS1 rearrangements. The results of GLORY are also consistent with findings from clinical trials. The reasons for this are considered to be as follows. One is that the identification of RET rearrangements is various by each study. Now, there is no gold-standard method for the identification of RET rearrangements. Available methods for RET testing have FISH, RT-PCR, or NGS. Consequently, some false RET-positive NSCLC patients might be included in previous studies, which led to the lower efficacy of RET inhibitors. Another is that the used targeted therapy is not selective RET inhibitor but multi-targeted RET inhibitors. Multi-targeted RET inhibitors may be not clinically enough RET kinase activity for patients with RET-rearranged NSCLC. Also, in-vitro experiments have proposed a signal switch as well as secondary RET mutations as possible mechanisms of resistance to RET inhibitors (18,19). Highly selective RET inhibitor and other therapeutic combinations with a RET inhibitor might help to improve clinical outcomes.
RET-rearranged NSCLC patients are rarely encountered. To identify clinicopathologic features and collect clinical data in a real-world setting of such patients, the global, multicenter registry such as GLORY is very useful. On the based the results of GLORY and several clinical trials, multi-targeted RET inhibitors have shown clinical antitumor activity in patients with RET-rearranged NSCLC. This results define RET rearrangement as a new molecular subgroup of NSCLC suitable for targeted therapy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research. The article did not undergo external peer review.
Conflicts of Interest: Dr. Yoh has received personal fees as honoraria from Chugai, Eli Lilly, AstraZeneca, Taiho, Boehringer Ingelheim, Bristol-Myers Squibb, and Ono. His institution has received research funding from Pfizer, Taiho, Eli Lilly, AstraZeneca, Bayer and Novartis.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998-2006. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [Crossref] [PubMed]
- Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell 1985;42:581-8. [Crossref] [PubMed]
- Ju YS, Lee WC, Shin JY, et al. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res 2012;22:436-45. [Crossref] [PubMed]
- Kohno T, Ichikawa H, Totoki Y, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med 2012;18:375-7. [Crossref] [PubMed]
- Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med 2012;18:378-81. [Crossref] [PubMed]
- Lipson D, Capelletti M, Yelensky R, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med 2012;18:382-4. [Crossref] [PubMed]
- Wang R, Hu H, Pan Y, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol 2012;30:4352-9. [Crossref] [PubMed]
- Gautschi O, Zander T, Keller FA, et al. A patient with lung adenocarcinoma and RET fusion treated with vandetanib. J Thorac Oncol 2013;8:e43-4. [Crossref] [PubMed]
- Falchook GS, Ordóñez NG, Bastida CC, et al. Effect of the RET Inhibitor Vandetanib in a Patient With RET Fusion-Positive Metastatic Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:e141-4. [Crossref] [PubMed]
- Drilon A, Wang L, Hasanovic A, et al. Response to Cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Discov 2013;3:630-5. [Crossref] [PubMed]
- Gautschi O, Milia J, Filleron T, et al. Targeting RET in Patients With RET-Rearranged Lung Cancers: Results From the Global, Multicenter RET Registry. J Clin Oncol 2017;35:1403-10. [Crossref] [PubMed]
- Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol 2016;17:1653-60. [Crossref] [PubMed]
- Yoh K, Seto T, Satouchi M, et al. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med 2017;5:42-50. [Crossref] [PubMed]
- Lee SH, Lee JK, Ahn MJ, et al. Vandetanib in pretreated patients with advanced non-small cell lung cancer-harboring RET rearrangement: a phase II clinical trial. Ann Oncol 2017;28:292-7. [PubMed]
- Huang Q, Schneeberger VE, Luetteke N, et al. Preclinical Modeling of KIF5B-RET Fusion Lung Adenocarcinoma. Mol Cancer Ther 2016;15:2521-9. [Crossref] [PubMed]
- Kang CW, Jang KW, Sohn J, et al. Antitumor Activity and Acquired Resistance Mechanism of Dovitinib (TKI258) in RET-Rearranged Lung Adenocarcinoma. Mol Cancer Ther 2015;14:2238-48. [Crossref] [PubMed]


