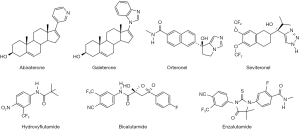
Androgen receptor antagonism and impact on inhibitors of androgen synthesis in prostate cancer therapy
In a recent article by Norris and colleagues, published in high-impact J Clin Invest (1), the authors investigated the potential role of androgen receptor (AR) antagonism and the efficacy of cytochrome P450 17A1 (hereafter called CYP17) inhibitors in prostate cancer models. The manuscript begins with a clear and thorough description of experimental/clinical candidate small molecules and clinically approved drugs designed as inhibitors of CYP17 (inhibitors of androgen synthesis) (2,3) or antiandrogens, agents that competitively antagonize the AR (4-6) (Figure 1), with the goal of treating castration resistant prostate cancer (CRPC). Of the promising CYP17 inhibitors, a clinical candidate, seviteronel (sev; VT-464) (Figure 1), shown to be a ‘C17,20-lyase selective inhibitor’, was also reported to have direct effects on AR function, although the molecular basis for this activity was not investigated (7).
The focus of this current report by the group led by Dr. McDonnell was to evaluate the potential AR antagonism activity of clinically relevant CYP17 inhibitors. In addition to the importance of the secondary activity to the efficacy of these inhibitors in cellular and animal models of prostate cancer, the author document that the ability of abiraterone (abi) and sev to inhibit CYP17 is dispensable for their efficacies against enzalutamide (enz)-resistant AR-F876L xenografts. Based on biochemical data and Phase 1clinical experience with sev, the authors evaluated the effects of sev and other well-known CYP17 inhibitors (Figure 1) on AR activity (1).
Using a whole-cell radioligand-binding assay, the investigators showed that unlike orteronel (ort), the other CYP17 inhibitors effectively displaced [3H]-R8118 (a potent AR antagonist) from the AR, albeit with varied potencies, some of which were comparable to the efficacies of the benchmark FDA-approved antiandrogens, hydroxyflutamide, bicalutamide (bic) and enz (Figure 1). In complementary AR transcriptional assays, the most potent AR antagonists, sev, galeterone (gal) and abi, which were further investigated, and were shown to effectively inhibit AR transactivation. These three inhibitors were also found to be equally efficacious progesterone receptor antagonists, but they did not impact glucocorticoid or mineralocorticoid receptor functions. These CYP17 inhibitors were also shown to be pure AR antagonists as they did not exhibit significant AR agonistic activities (no impact on reporter gene activity). Additional studies revealed that sev was as effective as enz in preventing androgen-mediated target gene expression, while gal and abi were less effective.
Using two complementary assays, the investigators showed that the CYP17 inhibitors did not deplete AR protein levels in LNCaP cells. However, because gal has been shown to induce degradation of AR in several in vitro and in vivo prostate cancer models by several independent groups (8-14), the results presented here should be treated with caution. Using LNCaP and VCAP prostate cancer cells, Norris et al. showed that the CYP17 inhibitions blocked androgen-mediated growth of AR-expressing prostate cancer cell lines, and also showed that the AR-negative DU-145 cell line was less responsive. We note that the authors fail to cite previous reports showing the effects of gal on the proliferation and inhibition of DU-145 and PC-3 cells and tumors (9,15).
It was shown that conformational change in the AR induced by the CYP17 inhibitors closely resembles the unliganded AR (apo-AR), suggesting that these compounds are mechanistically distinct from other classes of AR antagonists and would have clinical utility in the contexts in which existing antagonists have proven to be ineffective. The unique nature of these CYP17 inhibitors was further demonstrated using studies that validated their ability to prevent AR translocation to the nucleus and attenuating their interaction with target gene enhancers. Additional studies clearly demonstrated that the CYP17 inhibitors function as antagonist in AR-overexpressing CRPC models and that the inhibitors effectively antagonize the transcriptional activity of several clinically relevant AR mutants expressed in CRPC samples. Notably, the CYP17 inhibitor, ort, that lacked AR antagonistic activity, failed to inhibit the transcriptional activity of antiandrogen-resistant AR variants, even at concentrations up to 100 µM.
Studies using LNCaP cells engineered to overexpress AR-F876L (model of enz-resistant CRPC), showed that in contrast to the finding that enz was as effective as testosterone at activating AR target gene transcription, abi, gal and sev were ineffective. Additional studies in this model revealed that the CYP17 inhibitors did not stimulate AR-F876L nuclear accumulation and they also inhibited testosterone-induced recruitment of AR-F876L with AREs in the KLK and NKX3.1 genes. As expected, bic and enz treatment increased the proliferation of AR-overexpressing (LNCaP-AR) and enz-resistant (LNCaP-F876L) prostate cancer cells; however, the CYP17 inhibitors were without effects in either cell line. Collectively, these results led the authors to conclude that CYP17 inhibitors may have therapeutic utility in the management of CRPC patients who fail enz therapy due to selection for the AR-F876L mutation.
Finally, encouraged by these in vitro promising results and because of the importance of validation of efficacy using in vivo antitumor efficacy of lead drug candidates, in view of translation into the clinic, the authors assessed the efficacies of two CYP17 inhibitors (abi and sev) compared to enz using well-established hormone-sensitive and enz-resistant CRPC tumor xenograft models. Following the establishment of the effective dose of sev (100 mg/kg, twice daily) using the hormone-sensitive LNCaP xenograft models, the impact of sev and abi on the growth of enz-resistant LNCaP-F876L xenografts was determined. This later study was designed to isolate the CYP17-inhibitory activity from the AR antagonistic activity. Their data clearly showed that enz (30 mg/kg, once daily) had no effect on LNCaP-F876L tumor growth, and, neither did testosterone. In contrast, both abi and sev (each at 100 mg/kg, twice daily), significantly inhibited tumor growth (P<0.0001), regardless of testosterone administration. Importantly, the efficacy of sev was superior to that of abi, despite its lower molar dose, since the molecular weight of sev is 1.14-fold that of abi. In addition, analysis of plasma at termination of the study showed that the drug exposure was similar to that observed in patients. Together, the authors rightly concluded that the in vitro and importantly the in vivo antitumor efficacy data clearly support the role of AR antagonism in the mechanisms of action of the CYP17 inhibitors, sev, abi and gal, although gal was not assessed for its effects on tumor growth inhibition.
This is the first comprehensive head-to-head study of experimental/clinical candidate small molecules and clinically approved drugs that were designed as inhibitors of CYP17, with the strategy to validate the ability of some of these CYP17 inhibitors to also antagonize the AR. The authors are commended for their rigorous and comprehensive studies and report. The studies have validated previous studies by other research groups (2,12,16) and provides strong evidence to rationalize the clinical efficacies of “dual CYP17 inhibitors/AR antagonist” in the clinic in men with CRPC.
Although this report serves as a valuable proof-of-concept for the potential impact of ‘designed CYP17 inhibitors’ in the treatment of CRPC, additional studies would further enhance its potential impact. First, in the antitumor efficacy studies, given that the molar doses of sev and abi are 7.75- and 8.86-fold, respectively, higher than that of enz, at least one acceptable higher dose of enz should have been assessed in the study. Second, given that up-regulation of AR-V7 is a major mechanism of drug (enz and abi)-resistance in CRPC therapy, sev should have been tested in a CRPC model such as CWR22Rv1 with overexpressed AR-V7. Third, because of the high effective in vivo doses of sev and abi, and also because of AR antagonist association with convulsions/seizures (due to binding and activation of the central nervous system (CNS)-based GABAA receptor) (17,18), not only plasma, but also brain concentrations should have been determined to support the safety assessments of sev and abi. Indeed, assessment of the binding affinities of these agents to the GABAA receptor would also be valuable. Overall, given the current state of drug development for effective treatment of CRPC, the work should capture the interest of the wide prostate cancer therapy audience, and it is timely.
Acknowledgments
Funding: This work was supported in part by a grant from NIH and NCI (R21CA195694).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Peng Zhang (Department of Urology, Zhongnan Hospital of Wuhan University, Wuhan, China).
Conflicts of Interest: VC Njar is the lead inventor of Galeterone.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Norris JD, Ellison SJ, Baker JG, et al. Androgen receptor antagonism drives cytochrome P450 17A1 inhibitor efficacy in prostate cancer. J Clin Invest 2017;127:2326-38. [Crossref] [PubMed]
- Njar VC, Brodie AM. Discovery and development of Galeterone (TOK-001 or VN/124-1) for the treatment of all stages of prostate cancer. J Med Chem 2015;58:2077-87. [Crossref] [PubMed]
- Yin L, Hu Q. CYP17 inhibitors--abiraterone, C17,20-lyase inhibitors and multi-targeting agents. Nat Rev Urol 2014;11:32-42. [Crossref] [PubMed]
- Helsen C, Van den Broeck T, Voet A, et al. Androgen receptor antagonists for prostate cancer therapy. Endocr Relat Cancer 2014;21:T105-18. [Crossref] [PubMed]
- Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009;324:787-90. [Crossref] [PubMed]
- Vasaitis TS, Njar VC. Novel, potent anti-androgens of therapeutic potential: recent advances and promising developments. Future Med Chem 2010;2:667-80. [Crossref] [PubMed]
- Maity SN, Titus MA, Gyftaki R, et al. Targeting of CYP17A1 Lyase by VT-464 Inhibits Adrenal and Intratumoral Androgen Biosynthesis and Tumor Growth of Castration Resistant Prostate Cancer. Sci Rep 2016;6:35354. [Crossref] [PubMed]
- Bruno RD, Vasaitis TS, Gediya LK, et al. Synthesis and biological evaluations of putative metabolically stable analogs of VN/124-1 (TOK-001): head to head anti-tumor efficacy evaluation of VN/124-1 (TOK-001) and abiraterone in LAPC-4 human prostate cancer xenograft model. Steroids 2011;76:1268-79. [Crossref] [PubMed]
- Kwegyir-Afful AK, Bruno RD, Purushottamachar P, et al. Galeterone and VNPT55 disrupt Mnk-eIF4E to inhibit prostate cancer cell migration and invasion. FEBS J 2016;283:3898-918. [Crossref] [PubMed]
- Kwegyir-Afful AK, Ramalingam S, Purushottamachar P, et al. Galeterone and VNPT55 induce proteasomal degradation of AR/AR-V7, induce significant apoptosis via cytochrome c release and suppress growth of castration resistant prostate cancer xenografts in vivo. Oncotarget 2015;6:27440-60. [Crossref] [PubMed]
- Purushottamachar P, Godbole AM, Gediya LK, et al. Systematic structure modifications of multitarget prostate cancer drug candidate galeterone to produce novel androgen receptor down-regulating agents as an approach to treatment of advanced prostate cancer. J Med Chem 2013;56:4880-98. [Crossref] [PubMed]
- Soifer HS, Souleimanian N, Wu S, et al. Direct regulation of androgen receptor activity by potent CYP17 inhibitors in prostate cancer cells. J Biol Chem 2012;287:3777-87. [Crossref] [PubMed]
- Vasaitis T, Belosay A, Schayowitz A, et al. Androgen receptor inactivation contributes to antitumor efficacy of 17{alpha}-hydroxylase/17,20-lyase inhibitor 3beta-hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene in prostate cancer. Mol Cancer Ther 2008;7:2348-57. [Crossref] [PubMed]
- Yu Z, Cai C, Gao S, et al. Galeterone prevents androgen receptor binding to chromatin and enhances degradation of mutant androgen receptor. Clin Cancer Res 2014;20:4075-85. [Crossref] [PubMed]
- Bruno RD, Gover TD, Burger AM, et al. 17alpha-Hydroxylase/17,20 lyase inhibitor VN/124-1 inhibits growth of androgen-independent prostate cancer cells via induction of the endoplasmic reticulum stress response. Mol Cancer Ther 2008;7:2828-36. [Crossref] [PubMed]
- Richards J, Lim AC, Hay CW, et al. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100. Cancer Res 2012;72:2176-82. [Crossref] [PubMed]
- Clegg NJ, Wongvipat J, Joseph JD, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res 2012;72:1494-503. [Crossref] [PubMed]
- Foster WR, Car BD, Shi H, et al. Drug safety is a barrier to the discovery and development of new androgen receptor antagonists. Prostate 2011;71:480-8. [Crossref] [PubMed]