
Mixing, matching and modifying the prostate cancer microenvironment
Lu et al. (1) introduce a novel chimeric model of prostate cancer to interrogate the tumor microenvironment and signaling pathways in response to single targeting agents given alone or in combination with checkpoint inhibitors. This provides new preclinical data that reinforces clinical observations that combinatorial approaches for treating metastatic castration-resistant prostate cancer (mCRPC) may be more beneficial when compared with monotherapies (Figure 1). It also provides a rationale for the combination of biologic receptor and pathway targeting agents with the family of different checkpoint inhibitors. While the first demonstration of survival benefit by an immunotherapy for a solid tumor was in patients with minimally symptomatic or asymptomatic CRPC using an autologous dendritic cell product, sipuleucel-T (Provenge®) (3), immunotherapeutic approaches with single agent peptide or DNA vaccines using novel viral platforms, CAR T cells, and checkpoint inhibitors have all shown limited or minimal impact on the disease.
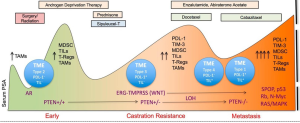
To date, it remains unclear as to the rationale for the suboptimal responses to checkpoint inhibitors in prostate cancer. Studies have suggested prostate cancer is not a hypermutated disease (4) compared with other genitourinary malignancies such as bladder and renal cancers, however, others have postulated that the absence of or lack of expression of PD1, PD-L1, or polymorphisms in molecules such as CTLA-4 may have some indistinct role. Despite these negative results, there are prostate cancer patients who have had dramatic, durable responses following treatment with ipilimumab alone or in combination with radiation therapy (5-7). Graff et al. (8) reported results from a pilot trial of prostate cancer patients with late disease and significant tumor burden demonstrating several dramatic responses when pembrolizumab was administered post enzalutamide failure, suggesting that the preclinical observations of enzalutamide (9) as an immune modulator may in fact be contributing to the response. The variations in responses to checkpoint inhibitors have now shown that not all cancers respond equally to the same checkpoint inhibitor and that a particular cancer may have unique responsiveness to a specific checkpoint drug.
In the “Letter” published by Lu et al. (1), the authors present data to confirm their hypothesis that a combination of immune checkpoint agents together with a targeted agent could affect myeloid-derived suppressor cells (MDSCs) in the setting of preserving normal T cell function. MDSCs contribute to an immune suppressive environment and have been implicated in cancer progression. However, how MDSCs respond to treatment and their role in providing a mechanism by the tumor microenvironment can be positively or negative influenced have not been completely studied. Clinically, MDSCs have been studied as potential biomarkers to assess response to treatment as well as disease progression. Preliminary retrospective data presented by Autio et al. (10) used a novel platform for a biomarker based assay in whole blood that enumerated MDSC from 36 patients with either metastatic castration sensitive prostate cancer (CSPC) and mCRPC. The results did not confirm any impact of chemotherapy on MDSCs nor were they significantly higher in those with visceral metastases, though a trend existed (20.3 vs. 24.0, P=0.076). There was a trend for higher MDSC values in patients with visceral metastases, which historically are associated with worse prognoses.
Lu et al. (1) tested the impact of novel immune oncology and biologic agents using a chimeric mouse model of mCRPC that could exhibit autochthonous tumor evolution. A novel non-germline mCRPC model in a C57BL/6 background was used via a JH61 and JH58 mouse embryonic stem cell clone. These were derived from several genotypes including the PB-Cre+PtenL/L smad4L/L mTmGL1+LSL-LUCL1+ genotypes. These animals developed metastases to lymph nodes and micrometastases in lungs and could provide an in vivo window into mechanism and response to therapies. As such, a panel of checkpoint inhibitors that have been shown to be safe and have a clinical signal in selected patients in early phase trials but did not impact on overall survival in phase III trials, have some measure of preclinical activity, and/or induce immunomodulation were studied. Among the drugs studied sere dasatinib (Sprycel®, a synthetic small molecule-inhibitor of SRC-family protein-tyrosine kinases), cabozantinib [Cometriq®, a small-molecule inhibitor of tyrosine kinases, including MET, VEGF receptors (VEGFRs), and AXL], BEZ235 [a phosphoinositide 3-kinase (PI3k)/mTOR dual inhibitor], along with anti-CTLA4 (Yervoy®) and anti-PD1 antibodies. These mCRPC-bearing chimeric mice received either checkpoint inhibitors alone or in combination with these drugs. As expected, the respected target agent monotherapies as well as the immune agents had minimal impact on the prostate tumor mass but the combination of cabozantinib and immune agent or BEZ plus immune agent showed potent synergistic efficacy both against the primary and metastatic lesions. Marked reduction of disease burden in addition to reduced proliferation and apoptosis were seen histologically. Dasatinib showed minimal activity when given in combination with a checkpoint drug. However, there was some impact on the disease as determined by a significant reduction of tumor-infiltrating lymphocytes (TILs) T cells suggesting impact on the tumor microenvironment. Depending on the murine model used, there was significant impact on the tumor microenvironment as assessed by a variety of signaling assays including phospho-receptor tyrosine kinase as well as cytokine assays. MDSCs showed a significantly higher sensitivity to cabozantinib and BEZ but not to dasatinib. In addition, when MDSCs were isolated from CRPC tumors treated with cytokines that were downregulated as a result of pretreatment of the tumor with cabozantinib or Bez, significant upregulation of Argl, Cybb, Ncf1 and Ncf4 were observed. The authors concluded that prostate cancer cells were capable of driving immunosuppression-related gene expression in MDSCs via the secretion of multiple cytokines. This was extrapolated further to suggest that there was paracrine signaling that was impaired by using cabozantinib or BEZ treatment.
These observations provide a real-time window into the interrogation of novel agents and their combinations with immune oncology drugs and provide further insight into the tumor microenvironment, the immune mechanisms at work, and the signaling pathways that are affected by drugs given singly or in combination with these immune agents. It may also explain mechanistically the lack of responsiveness to these single agents in prostate cancer. However, it does not completely explain why the majority of patients using single agent therapy fail and why individual patients may have durable responses. Nevertheless, it is a novel foothold by which the biology of these drugs may or may not show impact on the tumor microenvironment. Caution should be exercised that while preclinical models using novel drugs have often been successful in reducing or eliminating tumor burden, their use clinically may not similarly translate to comparable findings.
Despite these caveats, there are multiple studies that support the use of combinatorial approaches in prostate cancer many of which have been based on sound preclinical work. Ardiani et al. (9) studied the combination of drugs targeting the PI3k/Akt pathway and the androgen-receptor (AR) axis. They studied the combination of AZD5363, an adenosine triphosphate-competitive pan-Akt inhibitor and enzalutamide (9), an AR targeted drug was given at time of castration similarly and resulted in significant regression of tumors. The combination of AZD5363 and enzalutamide significantly delayed the development of resistance to enzalutamide in preclinical models via synergistic increases in cell cycle arrest as well as apoptosis. The authors support the idea that greater efficacy may result with earlier combination treatment.
In the TRAMP-C2 model, in vitro treatment with enzalutamide resulted in the up-regulation of MHC-I and Fas (11). Treatment with enzalutamide also induced a modest up-regulation of tumor antigens and cell-surface molecules in AR-expressing LNCaP human prostate carcinomas. Of note, enzalutamide or the AR-directed adrenal agent abiraterone when given in vitro, mediated major changes in several apoptotic genes in LNCaP cells. NAIP, a member of a family of inhibitors of apoptosis proteins that inhibit cell death via the inhibition of activated caspases was markedly down-regulated in LNCaP cells treated in vitro with enzalutamide (14-fold) or abiraterone (5-fold) (11). This family of inhibitory proteins has been shown to be overexpressed in a variety of malignancies and may contribute to the resistance of apoptosis, drug resistance, and tumor progression.
Other combinatorial approaches have evaluated sorafenib, a tyrosine kinase inhibitor along with enzalutamide in a CRPC model (12), enzalutamide combined with sorafenib decreased cell proliferation and induced apoptosis in the prostate cancer line, LNCaP. Tumor growth was suppressed in castrate-resistant LNCaP xenografts with the combination of these agents compared with each alone. While AR was down-regulated per Western blot, the ERK pathway was inhibited. Marques et al. (13). also using twelve human prostate cancer cells lines to study whether the combination of hormonal therapy with AZD5363 and AZD8186 could upregulate AR-target genes. The combination with hormonal therapy improved the efficacy and resulted in durable remissions. These data suggested that the combination resulted in upregulation of AR-target genes upon PI3k/Akt inhibition could result in efficacy via some form compensatory crosstalk between the AR and P13K/Akt pathways. Similar observations have been reported by Toren et al. (14) who also supported the premise of crosstalk between the PI3k/Akt/mTOR and RAF/MEK/ERK signaling pathways. Castration sensitive, castration resistant, and enzalutamide-resistant prostate cancer cell lines were treated with AZD5363, an Akt inhibitor and PD0325901, a MEK inhibitor, either alone or in combination. The authors confirmed that the co-targeting of these pathways showed that Akt inhibition induced apoptosis and inhibited cell growth in PTEN null cell lines; that MEK inhibition had a greater effect on the 22RV1 cells compared with AR-expressing LNCaP, or enzalutamide resistant cells. But there was synergy using Akt and MEK blockade in some of the cell lines but this was inconsistent among the cell lines studied.
These studies all serve to highlight the potential for combinatorial approaches for the treatment of prostate cancer. The chimeric models introduced by Lu et al. (1) provide a means to better explore in vivo the effects of combination drug and immune therapies. However, despite the usefulness of this approach, the overall heterogeneity (2) of prostate cancer continues to limit the rapidity by which preclinical success can translate into clinical implementation.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Peng Zhang (Department of Urology, Zhongnan Hospital of Wuhan University, Wuhan, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.08.42). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lu X, Horner JW, Paul E, et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 2017;543:728-32. [Crossref] [PubMed]
- Bryant G, Wang L, Mulholland DJ. Overcoming Oncogenic Mediated Tumor Immunity in Prostate Cancer. Int J Mol Sci 2017;18: [Crossref] [PubMed]
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411-22. [Crossref] [PubMed]
- Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013;499:214-8. [Crossref] [PubMed]
- Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol 2013;24:1813-21. [Crossref] [PubMed]
- Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:700-12. [Crossref] [PubMed]
- Beer TM, Kwon ED, Drake CG, et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J Clin Oncol 2017;35:40-47. [Crossref] [PubMed]
- Graff JN, Alumkal JJ, Drake CG, et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget 2016;7:52810-7. [Crossref] [PubMed]
- Ardiani A, Gameiro SR, Kwilas AR, et al. Androgen deprivation therapy sensitizes prostate cancer cells to T-cell killing through androgen receptor dependent modulation of the apoptotic pathway. Oncotarget 2014;5:9335-48. [Crossref] [PubMed]
- Autio KA, Wong P, Rabinowitz A, et al. Presence of myeloid-derived suppressor cells (MDSC) in patients with metastatic castration-sensitive and castration-resistant prostate cancer. J Clin Oncol 2015;33:Suppl 222.
- Toren P, Kim S, Cordonnier T, et al. Combination AZD5363 with Enzalutamide Significantly Delays Enzalutamide-resistant Prostate Cancer in Preclinical Models. Eur Urol 2015;67:986-90. [Crossref] [PubMed]
- Wu H, Zhang L, Gao X, et al. Combination of sorafenib and enzalutamide as a potential new approach for the treatment of castration-resistant prostate cancer. Cancer Lett 2017;385:108-16. [Crossref] [PubMed]
- Marques RB, Aghai A, de Ridder CM, et al. High Efficacy of Combination Therapy Using PI3K/AKT Inhibitors with Androgen Deprivation in Prostate Cancer Preclinical Models. Eur Urol 2015;67:1177-85. [Crossref] [PubMed]
- Toren P, Kim S, Johnson F, et al. Combined AKT and MEK Pathway Blockade in Pre-Clinical Models of Enzalutamide-Resistant Prostate Cancer. PLoS One 2016;11:e0152861 [Crossref] [PubMed]


