
Brigatinib, a new treatment option in ALK-rearranged advanced non-small cell lung carcinoma
Anaplastic lymphoma kinase (ALK) rearrangement is a rare feature in advanced non-small cell lung cancer (NSCLC), occurring in around 5% of the patients (1). This oncogenic addiction is more frequent in no/light-smokers, in thyroid transcription factor-1 (TTF1)-positive adenocarcinoma, with cribriform architecture and ring cells (2). ALK rearrangement—most of the time a translocation with a partner gene—induces the formation of a fusion protein with oncogenic properties. The first targeted therapy developed for ALK-rearranged NSCLC was crizotinib, an ALK tyrosine kinase inhibitor (TKI). Crizotinib was proved to be superior to chemotherapy in first-line setting in ALK-rearranged NSCLC, with an overall response rate (ORR) of 74% and a progression-free survival (PFS) of 10.9 months (3). Crizotinib in first-line treatment is now widely used. Other ALK TKIs have been developed, as brigatinib. Results of the phase I-II trial published in 2016 showed in 79 advanced NSCLC patients treated with brigatinib an ORR at 75% [72% in previously crizotinib treated patients (n=71)] and a PFS (median) of 13.2 months (4). Safety profile showed specific lung toxicity, occurring at the beginning of the treatment (median 2 days) in 8% of the patients, with incidence increasing with the dose of brigatinib. The ALTA trial, published in May 2017 in Journal of Clinical Oncology by Kim et al. was a phase II trial that randomized patients with advanced ALK-rearranged NSCLC between 2 doses of brigatinib (90 mg daily and 90 mg daily for 7 days then 180 mg daily) (5). The primary endpoint was ORR, and no comparison between the 2 arms was pre-planned. Assuming an ORR ≥35% with a power of 90% and a bilateral alpha risk at 0.025, the study had to include 109 patients per arm. Patients should have an advanced ALK-rearranged NSCLC with progression with crizotinib and a performance status (PS) between 0 and 2. Brain metastases were allowed only if asymptomatic and with stable doses of steroid treatment. Two-hundred and twenty-two patients were randomized [112 patients arm A (90 mg) and 110 patients arm B (90 mg then 180 mg)]. Both arms were well-balanced, with a median age at 54 years, 57% of women, 31% of Asian, 60% of non-smokers, 97% of adenocarcinoma, 69% of brain metastases. ORR (investigator-assessed) was 45% in arm A and 54% in arm B, and disease-control rate was 82% (arm A) and 86% (arm B). Median time to response was 1.8 months (arm A) and 1.9 months (arm B), and duration of response was 13.8 months (arm A) and 11.8 months (arm B). Median PFS was 9.2 months (arm A) and 12.9 months (arm B). Post-hoc comparison between the 2 arms for PFS showed a clear benefit for arm B, with a hazard ratio (HR) at 0.55 [95% confidence interval (CI), 0.36–0.86]. One-year overall survival (OS) probability was 71% (arm A) and 80% (arm B). As shown in the phase I-II trial, brigatinib had intracranial efficacy. ORR for measurable brain metastases was 42% (arm A, n=26) and 67% (arm B, n=18). For active brain metastases (i.e., metastases without previous brain radiotherapy, or progression after brain radiotherapy), intracranial ORR was 42% (arm A, n=19) and 73% (arm B, n=15). Intracranial PFS (median) was 15.6 months (arm A) and 12.8 months (arm B), with a duration of intracranial response (median) not reached in both arms. In term of safety, no new signal was detected. In both arms, main toxicities (≥10% patients) were nausea (33%/40%), diarrhea (19%/38%), vomiting (24%/23%), headache (28%/27%), decrease appetite (22%/15%) and hypertension (11%/21%). Grade 3–4 toxicities were rare: hypertension (6%/6%), increased creatine phosphokinase (3%/9%) and rash (1%/3%), mainly. Pulmonary early toxicity occurred in 6% of patients (n=14), during the first 2 days of treatment (always at 90mg daily of brigatinib). Grade 3–5 pulmonary toxicities concerned 3% of patients (n=7). Half of the patients (n=7) had successfully continued brigatinib after suspension, whereas 7 patients stopped brigatinib definitely. One patient died from acute respiratory failure (<1%). Multivariate analysis showed that advanced age [odds ratio (OR) =2.10; 95% CI, 1.21–3.65] and time from last crizotinib dose to first brigatinib dose less than 7 days (OR =3.88; 95% CI, 1.10–13.68) were associated with early pulmonary toxicity. Seven percent (arm A) and 20% (arm B) patients had dose reduction, due to increase of creatine phosphokinase, pneumonitis and rash.
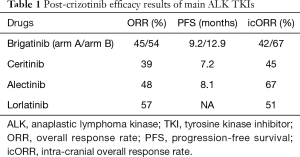
In summary, the ALTA trial is a positive study, with results suggesting high efficacy of brigatinib after failure of crizotinib in ALK-rearranged advanced NSCLC. Brigatinib has also good brain penetration and intracranial efficacy. Toxicity seems manageable, paying attention to the specific dose-dependent early pulmonary toxicity, rare but sometimes severe, and suggesting the use of a two-step initiation of brigatinib (90 mg one week then 180 mg). Will these results change our treatment strategy of ALK-rearranged NSCLC? Brigatinib appears clearly as a good treatment option after progression with crizotinib. Other validated ALK TKIs in this second-line setting are ceritinib and alectinib. Ceritinib has proved its efficacy after crizotinib treatment. The ASCEND-2 phase II trial, testing ceritinib after crizotinib showed an ORR at 39%, a median PFS at 7.2 months, and intracranial ORR at 45% (6). For alectinib, in the same setting, ORR was 48%, median PFS 8.1 months, and intracranial ORR 67% (7). Even if comparison between these 3 studies is subject to caution, brigatinib seems to have slightly longer PFS (median 12.9 months with 180 mg per day). Concerning intracranial efficacy, both brigatinib and alectinib reach acceptable ORR, whereas ceritinib allowed only intracranial ORR less than 50%, leading probably to choose rather brigatinib or alectinib in case of brain progression after crizotinib. A fourth ALK TKI, lorlatinib, has also an accelerated development and should receive a Food and Drug Administration (FDA) approval soon. In the phase I-II trial, ORR with lorlatinib given after crizotinib was 57% (n=7) and intracranial ORR for target lesions (≥5 mm; no prior radiotherapy or progression post prior radiotherapy) was 51% (n=35) (8). Beyond, these efficacy data (Table 1), the choice between these drugs will probably be conditioned by several factors. First, concerning the toxicity profile, brigatinib seems to be better tolerated than ceritinib. In the ASCEND-2 trial, nausea and diarrhea occurred in 80% of patients (6% grade 3–4), vomiting in 63% (4% grade 3–4) and ALT elevation in 44% (17% grade 3–4) (6). In the opposite way, alectinib and lorlatinib showed very good safety profile (7,8). The last point is the difference of activity of ALK TKIs depending of the ALK mutational status at progression under crizotinib. Preliminary studies have shown that in around one-third of the cases, secondary resistance to crizotinib was an ALK-dependent process, mainly due to the acquisition of an ALK resistance mutation (9). Based on primary NSCLC cultures, ALK TKIs had various efficacy depending on the type of the mutation (9). Of interest, brigatinib seemed to have the best efficacy in vitro in case of high-resistance G1202R mutation, compared to crizotinib, ceritinib and alectinib (10). In the ALTA trial, one patient with G1202R mutation had a partial response with brigatinib. However, larger clinical data with molecular status at progression on ALK TKIs (sequential re-biopsies) are urgently needed to correlate with drug efficacy and draw definitive conclusions.
Full table
These considerations will evolve in the very near future, as the treatment strategy is quickly changing in these patients. Several recent results on second-generation ALK TKIs use in first-line setting have been published. The ASCEND-4 trial, testing ceritinib in first-line, showed a benefit compared to standard platinum-based chemotherapy (11). However, beyond the poor safety profile of ceritinib, the PFS of 16.6 months in this trial was not very different from the cumulated PFS of crizotinib (10.9 months) and ceritinib in second-line (7.2 months) based on the results of PROFILE-1014 and ASCEND-2 trials (3,6), questioning the interest of ceritinib in front-line treatment. The ALEX trial compared alectinib to crizotinib in first-line treatment, and showed a large benefit of alectinib over crizotinib, with a median PFS not reached with alectinib, versus 11.1 months with crizotinib (HR =0.47; 95% CI, 0.34–0.65) (12). In the same setting, median PFS in Asiatic population (J-ALEX trial) was 25.9 months with alectinib, versus 10.2 months with crizotinib (13). This important difference will probably set alectinib as the preferred choice of treatment for advanced ALK-rearranged NSCLC as first-line treatment until publication of the results on other ALK TKIs in first-line treatment. For brigatinib, the phase III comparing brigatinib and crizotinib is ongoing (NCT02737501), and the amplitude of benefit with brigatinib, if present, will be determinant for the positioning of this drug in first-line. Unsolved questions remain, like the efficacy of brigatinib after alectinib, the change in the natural history of the disease with the prescription of new generation ALK TKI in first-line setting, as preliminary data suggest that the type and proportion of ALK mutations vary with the drugs (9).
In conclusion, brigatinib is a new treatment option after crizotinib progression in ALK-rearranged NSCLC, with good efficacy, notably for brain metastases. Its good safety profile and putative efficacy in case of high-resistance G1202R ALK mutation seem particularly interesting. The current development of other ALK TKI, as entrectinib (14), will enrich the strategy treatment, with a necessity of a serial molecular screening to select the right treatment at the good moment. But the availability of a large number of ALK TKIs, allowing multiple sequential treatments, is the main determinant for prolonged survival in these patients (15).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Wei Xu (Jiangsu Provincial Key Laboratory of Geriatrics, Department of Geriatrics, the First Affiliated Hospital of Nanjing Medical University, Nanjing, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.09.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016;387:1415-26. [Crossref] [PubMed]
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Gettinger SN, Bazhenova LA, Langer CJ, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol 2016;17:1683-96. [Crossref] [PubMed]
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol 2017;35:2490-8. [Crossref] [PubMed]
- Crinò L, Ahn MJ, De Marinis F, et al. Multicenter Phase II Study of Whole-Body and Intracranial Activity With Ceritinib in Patients With ALK-Rearranged Non-Small-Cell Lung Cancer Previously Treated With Chemotherapy and Crizotinib: Results From ASCEND-2. J Clin Oncol 2016;34:2866-73. [Crossref] [PubMed]
- Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 2016;17:234-42. [Crossref] [PubMed]
- Shaw AT, Ou SI, Felip E, et al. Efficacy and safety of lorlatinib in patients (pts) with ALK+ non-small cell lung cancer (NSCLC) with one or more prior ALK tyrosine kinase inhibitor (TKI): A phase I/II study. J Clin Oncol 2017;35:abstr 9006.
- Gainor JF, Dardaei L, Yoda S, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- Zhang S, Anjum R, Squillace R, et al. The Potent ALK Inhibitor Brigatinib (AP26113) Overcomes Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in Preclinical Models. Clin Cancer Res 2016;22:5527-38. [Crossref] [PubMed]
- Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK -Positive Non–Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Takiguchi Y, Hida T, Nokihara H, et al. Updated efficacy and safety of the j-alex study comparing alectinib (ALC) with crizotinib (CRZ) in ALK-inhibitor naïve ALK fusion positive non-small cell lung cancer (ALK+ NSCLC). J Clin Oncol 2017;35:abstr 9064.
- Drilon A, Siena S, Ou SI, et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov 2017;7:400-9. [Crossref] [PubMed]
- Duruisseaux M, Besse B, Cadranel J, et al. Overall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): a French nationwide cohort retrospective study. Oncotarget 2017;8:21903-17. [PubMed]


