
Targeted therapies of HER2-positive gastric adenocarcinoma
Worldwide, gastric cancer is the fifth common cancer type and causes the third most cancer-associated deaths (1), mostly due to its commonly fatal outcome especially in locally advanced or metastatic disease. For over a decade, standard of care in metastatic disease was based on chemotherapy doublets or triplets containing platinum agents plus fluoropyrimidine in combination with either taxanes or anthracyclines (2). Molecular subtyping of gastric adenocarcinoma has identified promising protooncogenes as novel therapeutic targets (3). One of them is the human epidermal growth factor receptor 2 (HER2/ERBB2), detected in a subset of roughly 9% to 18% of gastric adenocarcinomas (4).
HER2-overexpression in gastric cancer was mostly reported to be associated with reduced overall survival (OS), yet few studies show no effect on prognosis or even rebut this finding. In contrast to breast cancer, HER2-positivity in gastric cancer might still have other prognostic implications (4).
Following the 2010 published data of the ToGA trial, HER2-targeting monoclonal antibody trastuzumab was granted first line approval for advanced, HER2 overexpressing gastric adenocarcinoma. A combination chemotherapy of cisplatinum plus capecitabine or 5-fluorouracil was complemented by trastuzumab in the verum test cohort (5). Herein an OS benefit of 2.7 months was shown for the trastuzumab-treated patients. Post hoc analyses of more stringent inclusion criteria, either (I) HER2 immunohistochemistry (IHC) staining level 3+ or (II) IHC 2+ and HER2 fluorescence in situ hybridization (FISH) positivity, revealed the most appropriate treatment cohort, resulting in a 4.2 months prolonged survival for the trastuzumab treated patients (5).
Recently data from the phase II/III GATSBY trial in second line treatment of HER2-positive gastric adenocarcinoma were published (6). Next to evaluation of an adequate trastuzumab emtansine dosage, the antibody-drug conjugate was tested against physicians choice docetaxel or paclitaxel, primarily measured in OS rates. Though trastuzumab emtansine was previously reported to show benefit in HER2-positive pretreated breast cancer (7), in gastric adenocarcinoma beneficial survival for the antibody-drug conjugate could not be reported (median OS 7.9 months for trastuzumab emtansine vs. 8.6 months for taxane, P=0.86). Yet, in case of tolerability, the more specific treatment showed fewer and less intense side effects (6). First line combination therapy of trastuzumab emtansine plus capecitabine (NCT01702558) is still being evaluated.
Lately, data from the TRIO-013/LOGiC placebo controlled phase III trial in advanced gastric adenocarcinoma were published (8). This study conducted capecitabine plus oxaliplatin (CapeOx) regimen randomly added by lapatinib or placebo in n=545, thereof n=487 centrally confirmed HER2 FISH-positive, patients. While its primary endpoint OS in the latter cohort was not met (12.2 months OS in the lapatinib cohort vs. 10.5 months OS in the placebo cohort, P=0.3492), progression free survival (PFS) was significantly prolonged by 0.6 months adding lapatinib to CapeOx (P=0.0381). Furthermore overall response rate to therapy was higher with than without lapatinib (53% vs. 39%, P=0.0031) (8). In post hoc descriptive analyses, interestingly OS improvement for lapatinib treated patients was found for Asians (P=0.0261) and those under 60 years of age (P=0.0141) (8), suggesting possible detrimental effects of age and diverging tumor’s biological behavior regarding the patients origin. Upon a closer look at lapatinib plus CapeOx safety profile serious adverse events (AE) were only slightly higher, yet fatal AE’s were 6% in the lapatinib arm and 3% in the placebo arm. Most relevant AE in the CapeOx plus lapatinib arm were diarrhea (any grade 58% vs. 29%) and vomiting (any grade 44% vs. 36%). Among others, fatigue, peripheral neuropathy, asthenia, neutropenia, anemia or ascites were of comparable incidence (8). Lapatinib is a small molecule drug and acts as an intracellular disruptor of HER2 downstream signaling. Therefore it seems legitimate to test patient’s HER2 status preferably by FISH analysis. Especially with the awareness of the Asian TyTAN trial (9), it must be questioned why regular IHC evaluations, as proposed by the FDA before starting extracellular monoclonal antibody therapy with trastuzumab (IHC 3+ plus FISH positivity and IHC 2+ plus FISH positivity) (4) were not relevantly evaluated in this study.
The phase II GastroLab trial in second line treatment of gastric adenocarcinoma with lapatinib vs. lapatinib plus capecitabine was performed by German AIO society (10). This study also failed to show beneficial effects on survival. Yet, response rates to therapy were significantly higher in combination therapy of capecitabine plus lapatinib compared to lapatinib alone. Due to these findings, lapatinib seems to improve response to anti-neoplastic therapy but does not promise a durating treatment approach for gastric adenocarcinoma solely.
A series of molecular resistance mechanisms against lapatinib (and trastuzumab) have been identified and are depicted in simplified downstream model of HER2-activation in Figure 1.
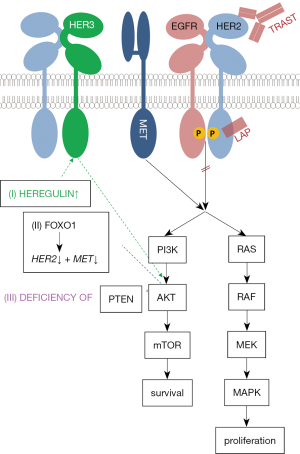
- Heregulin is an activator of HER3-activation. Lapatinib use consecutively leads to heregulin liberation and HER3-dependent AKT activation (12).
- AKT-driven downregulation of Forkhead box protein O1 (FOXO1) indirectly leads to an overexpression MET and HER2. As for the treatment with HER2 antagonists, tumor’s proliferative activity must be regulated towards HER2-independend MET downstream signaling (13,15).
- Loss or deficiency of phosphatase and tensin homolog (PTEN) leads to reduced inhibition and an unproportional activation via phosphoinositol-3-kinase (PI3K) of AKT downstream signaling (14).
Future therapeutic approaches should address these mechanisms of resistance by possibly adding HER3- (as performed in NCT01602406 trial), PI3-kinase-, or MET-antagonists to lapatinib. Another promising approach in in HER2-positive gastric adenocarcinoma is the concomitant enhancement of patient’s T-cellular response. As in many solid tumors, PD-1 blockade (phase Ib/II trial, NCT02901301) seems to be a well-tolerated treatment approach. Beyond, a HER2/CD3-bispecific monoclonal antibody (16) was built to contact tumor cells with CD3+ T-cells (phase I trial, NCT02829372).
In conclusion, HER2-targeted therapy is a relevant option in IHC- and FISH-positive gastric adenocarcinoma. In contrast to trastuzumab, lapatinib was not able to improve outcome of HER2-positive gastric cancer patients but emphasizes response rates in cytotoxic combination therapy. Lapatinib seems to be suitable especially for a subset of patients (i.e., <60 years of age and Asian origin). Resistance mechanisms against lapatinib have been reported and might be overcome by adding further targeted therapies to HER2-antagonizing combination therapies. In contrast to breast cancer, HER2-targeting in gastric adenocarcinoma seems to be more challenging. Therefore treatment strategies such as the enhancement of T-cellular activity in the tumor’s microenvironment are focus of recruiting studies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Hongcheng Zhu (Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.09.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Wagner AD, Grothe W, Haerting J, et al. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006;24:2903-9. [Crossref] [PubMed]
- Ranzani GN, Pellegata NS, Previderè C, et al. Heterogeneous protooncogene amplification correlates with tumor progression and presence of metastases in gastric cancer patients. Cancer Res 1990;50:7811-4. [PubMed]
- Jørgensen JT. Role of human epidermal growth factor receptor 2 in gastric cancer: Biological and pharmacological aspects. World J Gastroenterol 2014;20:4526-35. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol 2017;18:640-53. [Crossref] [PubMed]
- Krop IE, Kim SB, González-Martín A, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): A randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:689-99. [Crossref] [PubMed]
- Hecht JR, Bang YJ, Qin SK, et al. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC--A Randomized Phase III Trial. J Clin Oncol 2016;34:443-51. [Crossref] [PubMed]
- Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol 2014;32:2039-49. [Crossref] [PubMed]
- Lorenzen S, Riera Knorrenschild J, Haag GM, et al. Lapatinib versus lapatinib plus capecitabine as second-line treatment in human epidermal growth factor receptor 2-amplified metastatic gastro-oesophageal cancer: a randomised phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Eur J Cancer 2015;51:569-76. [Crossref] [PubMed]
- Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 2006;7:505-16. [Crossref] [PubMed]
- Nonagase Y, Yonesaka K, Kawakami H, et al. Heregulin-expressing HER2-positive breast and gastric cancer exhibited heterogeneous susceptibility to the anti-HER2 agents lapatinib, trastuzumab and T-DM1. Oncotarget 2016;7:84860-71. [PubMed]
- Park J, Choi Y, Ko YS, et al. FOXO1 Suppression is a Determinant of Acquired Lapatinib-Resistance in HER2-Positive Gastric Cancer Cells Through MET Upregulation. Cancer Res Treat 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Zhang X, Park JS, Park KH, et al. PTEN deficiency as a predictive biomarker of resistance to HER2-targeted therapy in advanced gastric cancer. Oncology 2015;88:76-85. [Crossref] [PubMed]
- Zhang Z, Wang J, Ji D, et al. Functional genetic approach identifies MET, HER3, IGF1R, INSR pathways as determinants of lapatinib unresponsiveness in HER2-positive gastric cancer. Clin Cancer Res 2014;20:4559-73. [Crossref] [PubMed]
- Lopez-Albaitero A, Xu H, Guo H, et al. Overcoming resistance to HER2-targeted therapy with a novel HER2/CD3 bispecific antibody. Oncoimmunology 2017;6:e1267891 [Crossref] [PubMed]


