
Magnolol inhibits tumor cell growth in human pancreatic cancer cell
Introduction
Pancreatic ductal adenocarcinoma is the seventh most lethal malignancy (1) with a high mortality rate owing to its invasiveness, rapid progression, and resistance to treatment. Given the particular anatomical location and biological characteristics, 90% of pancreatic cancer cases exhibit distant metastasis at the time of initial diagnosis such that surgical resection is no longer an option (2). Clinical studies have reported that gemcitabine can be used as a first-line treatment to reduce pain and relieve symptoms, thereby improving the quality of life of patients and prolonging their survival (3). Nonetheless, gemcitabine chemotherapy has a 5-year survival rate of only 1–3% with a median survival of 3–5 months, as well as toxic side effects including vomiting, nausea, diarrhea, neutrophil reduction, and hand-foot syndrome (4). As such, there is an urgent need to identify alternatives to conventional chemotherapeutic agents that are toxic to pancreatic cancer cells but do not have side effects.
Magnolol is a diallyl diphenyl compound extracted from the bark, roots, and stems of Magnolia officinalis that is often used in traditional Chinese medicine (5,6). Magnolol has anti-microbial, -inflammatory, -oxidant, -coagulant, and -tumor effects and has been shown to alleviate inflammatory pain (7-9). The anti-tumor effects of magnolol have been demonstrated in many cancer cell types, including melanoma, prostate cancer, colon cancer, liver cancer, and leukemia cells (10-14). Magnolol was found to act as a tumor suppressor in SGC-7901 human gastric cancer cells by inhibiting the phosphorylation of phosphoinositide 3-kinase, resulting in a decrease in the level of phosphorylated Akt, an increase in the expression of B cell lymphoma (Bcl)-associated X protein with concomitant reduction in the level of the anti-apoptotic protein Bcl-2, and activation of caspase-3-induced apoptosis (15). Magnolol was also shown to inhibit the growth of MDA-MB-231 and MCF-7 breast cancer cells and suppress invasion, adhesion, and metastasis of MDA-MB-231 cells (16). However, the effects and mechanism of action of magnolol in human pancreatic ductal adenocarcinoma are unknown.
To address this issue, the present study investigated the anti-tumor effects of magnolol in four human pancreatic cancer cell lines. We found that magnolol inhibited cell proliferation and migration, and also induced apoptosis. Furthermore, the inhibition of migration by magnolol may mediate via inhibition of cathepsin D (CTSD) expression.
Methods
Reagents
Magnolol (purity: 98%) was purchased from Melonepharma Biotechnology (Dalian, China) and dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) to obtain a stock solution of 100 mM that was stored at −20 °C until use. Gemcitabine hydrochloride for injection was purchased from Hansoh Pharmaceutical (Nanjing, China) and dissolved in saline to obtain a stock solution of 80 µM that was stored at −20 °C. Anti-CTSD antibody was purchased from Abcam (Cambridge, MA, USA).
Cell culture
PANC-1, CFPAC-1, ASPC-1, and MIAPaCa-2 human pancreatic cancer cell lines were obtained from the American Type Cell Collection (Manassas, VA, USA). Cells were cultured at 37 °C and 5% CO2 in Dulbecco’s Modified Eagle’s Medium (DMEM; Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich).
Cell viability assay
Cell proliferation was evaluated with Cell Counting Kit-8 (CCK-8) (Beyotime Institute of Biotechnology, Shanghai, China) according to the manufacturer’s instructions. Briefly, cells (2×103/well) were seeded in 96-well plates. After 24 h, the cells were treated with magnolol, or vehicle (DMSO) for 48 h. A 10-µL volume of CCK-8 was added to each well followed by incubation for 4 h. The absorbance at 450 nm was then measured with a microplate reader (Power WaveXS2; Biotek, Winooski, VT, USA). Three replicates were prepared for each experimental condition. The inhibition of cell growth was determined based on the difference in optical density between experimental and control wells.
Cell cycle and apoptosis analysis by flow cytometry
Cell apoptosis of magnolol-treated cells were analyzed by flow cytometry. To evaluate apoptosis, cell pellets were incubated in Annexin V-fluorescein isothiocyanate and PI buffer (Jiangsu KeyGEN Biotech, Nanjing, China) at room temperature for 20 min in the dark and then sorted on a flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Annexin V-positive/PI-negative and double-stained cells were recorded as early and late apoptotic cells, respectively. The DNA content of cells was determined on a FACS Calibur flow cytometer using Cell Quest software.
Hoechst staining
Cells were treated for 48 h with medium only or magnolol at concentrations up to 100 µM, and apoptotic cells were detected by Hoechst staining. Cells were first fixed with 4% paraformaldehyde (Kelong, Chengdu, China) for 30 min at room temperature, stained with Hoechst (Beyotime Institute of Biotechnology) for 20 min, and visualized under a fluorescence microscope (DM4000B; Leica, Wetzlar, Germany).
Wound healing and migration assays
Cells (2×105/well) were seeded in a 6-well plate with the medium containing 10% FBS for 24 h. When cells were confluent, the plate was scratched with a pipette tip; the cells were then washed with PBS and treated with magnolol or vehicle (DMSO) for 48 h, and the medium containing 1% FBS was exchanged. The width of the wound was evaluated under a light microscope.
The cell migration assay was carried out with transwell chambers containing 8-µm pore inserts (Corning, Midland, MI, USA). Cells were resuspended in DMEM containing 0.1% bovine serum albumin (Beyotime Institute of Biotechnology) and 2×105 cells in 100 µL medium were seeded in the upper chamber. DMEM with 10% FBS was added to the lower chamber. After incubation for 24 h, non-migrated cells were removed with a cotton swab. The transwell membranes were fixed with 4% paraformaldehyde for 30 min, and stained with Crystal Violet for 20 min. Cells that had migrated to the lower surface of the membrane were counted under a light microscope.
Western blotting
Cells (5×105/well) were seeded in 6-well plates for 24 h. After culturing with magnolol or gemcitabine for 48 h, cells were collected and then resuspended in radioimmunoprecipitation lysis buffer (Beyotime Institute of Biotechnology). The protein content of the lysates was determined with the Bicinchoninic Acid Protein Assay kit (Beyotime Institute of Biotechnology). Proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA) that was blocked for 2 h at room temperature in Tris-buffered saline containing 0.1% Tween-20 and 5% nonfat milk. The membrane was then probed overnight at 4 °C with an anti-CTSD antibody (1:1000), followed by a horseradish peroxidase-conjugated secondary antibody. Protein bands were visualized by enhanced chemiluminescence (GE Healthcare, Uppsala, Sweden).
RNA interference
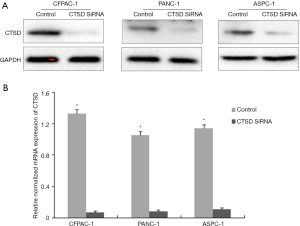
ASPC-1 cells were cultured at 37 °C and 5% CO2 in DMEM supplemented with 10% FBS for 24 h, then transfected with short interfering (si)RNA containing the CTSD target sequence (5'-GGC UCU GGA UAC AGU GCU CTT-3' and 5'-GAG CAC UGU AUC CAG AGC CTT-3') or control siRNA (Invitrogen, Carlsbad, CA, USA). After 48 h, the efficiency of CTSD knockdown was evaluated by western blotting and quantitative real-time (qRT)-PCR. Cells with CTSD knockdown were used for the cell migration assay.
qRT-PCR
CTSD mRNA expression was examined by qRT-PCR. Total RNA was extracted from cells with the TRIzol Plus RNA Purification kit (Invitrogen), and qRT-PCR was carried out using a 10-µL reaction mixture comprising 5 µL SYBR Select Master mix (Invitrogen), 1 µL of each primer, and 3 µL of cDNA template. Reactions were carried out in a 96-tube plate on a CFX96 real-time system (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Data are expressed as mean ± standard deviation. Experiments were performed at least three times. Statistical analyses were carried out using SPSS for Windows v.13.0 software (SPSS Inc., Chicago, IL, USA). Differences between groups were evaluated by one- or two-way analysis of variance. P<0.05 was considered statistically significant.
Results
Magnolol suppresses proliferation of pancreatic cancer cells
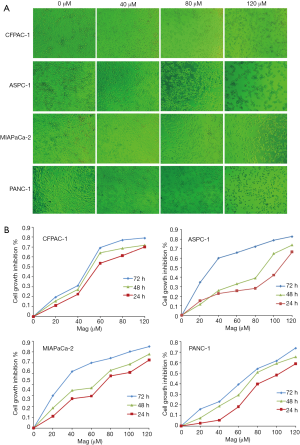
We first examined the growth-inhibitory effect of magnolol on human pancreatic cancer cell lines. Cells treated with magnolol (20–120 µM) showed alterations in morphology relative to vehicle (DMSO)-treated cells; that is, the cells were shrunken, round, fragmented, and became detached from the substratum with increasing concentrations of magnolol (Figure 1A). Magnolol induced concentration- and time-dependent growth inhibition in all four cell lines, as determined by the CCK-8 assay (Figure 1B).
Magnolol induces apoptosis in pancreatic cancer cells
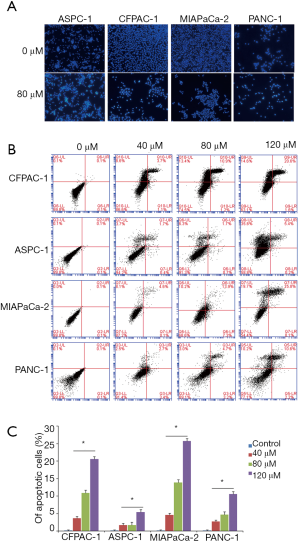
Magnolol induced cell apoptosis in a dose- and time-dependent manner, as determined by Hoechst staining and flow cytometry (Figure 2). The fraction of cells with apoptotic bodies was greater in magnolol-treated as compared to control cells (Figure 2A). The rate of apoptosis ranged from 1.7% to 4.6%, from 1.7% to 13.9%, and from 5.4% to 25.8% upon treatment with 40, 80, and 120 µM magnolol, respectively (Figure 2B).
Magnolol inhibits pancreatic cancer cell migration
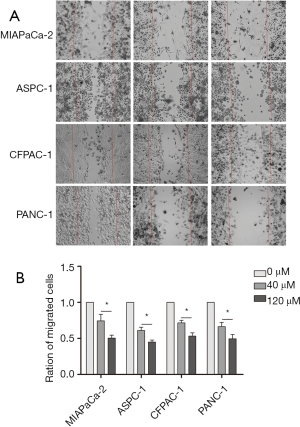
Pancreatic cancer cells were treated with high and low concentrations of magnolol and cell migration was evaluated with the wound healing assay. Wound size was larger in cells treated with 120 as compared to 40 µM magnolol and was larger in magnolol-treated than in control cells (Figure 3). Thus, magnolol suppresses pancreatic cancer cell migration in a concentration-dependent manner.
Regulation of CTSD by magnolol
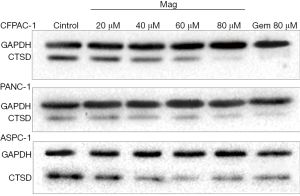
CTSD is an aspartic endoprotease that is overexpressed in many malignancies and it plays an important role in tumor cell proliferation, invasion, metastasis, and angiogenesis (17). We examined whether the magnolol alters CTSD expression in PANC-1, ASPC-1, and CFPAC-1 cells by western blotting following treatment with magnolol or vehicle (DMSO) for 72 h. CTSD expression was decreased in a concentration-dependent manner in the presence of magnolol in all three cell lines, although levels were similar in cells treated with 80 µM magnolol and 80 µM gemcitabine (Figure 4). These results indicate that magnolol negatively regulates CTSD expression in pancreatic cancer cells.
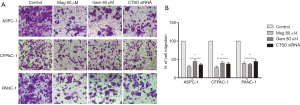
CTSD knockdown inhibits pancreatic cancer cell migration
Given our observation that magnolol inhibited cell migration, and also inhibited CTSD expression in pancreatic cancer cells, we speculated that CTSD expression is related to cell migration. We therefore investigated the effects of siRNA-mediated CTSD knockdown on cell migration with the transwell assay (Figure 5). CTSD knockdown suppressed migration relative to control siRNA treatment in PANC-1, ASPC-1, and CFPAC-1 cells, which phenocopied the migration defects observed in cells treated with magnolol or gemcitabine (Figure 6). These results suggest that magnolol inhibits pancreatic cancer cell migration may through negative regulation of CTSD.
Discussion
Magnolol is used in Chinese medicine to treat gastrointestinal disorders, cough, anxiety, and anaphylaxis. A previous study reported that magnolol inhibited angiogenesis to suppress tumor growth (18), and magnolol derivatives inhibited proliferation and metastasis in PC-3 prostate cancer cells by inducing autophagy and blocking tumor angiogenesis (19). Magnolol was also shown to promote apoptosis in prostate cancer cells by activating p38 mitogen-associated protein kinase, resulting in the release of tumor necrosis factor-α and upregulation of matrix metalloproteinase (MMP)-9 expression (20). In this study, we found that magnolol treatment inhibited proliferation and migration and induced apoptosis in four pancreatic cancer cell lines while negatively regulating CTSD expression.
CTSD is an aspartic endoprotease that is distributed in lysosomes where it acts as a protein-degrading enzyme (21). Under normal circumstances, CTSD contributes to protein metabolism and hormone and antigen processing by degrading extracellular matrix and other proteins. Moreover, cytoplasmic concentrations of CTSD are typically very low (22). However, it was reported that CTSD is overexpressed and secreted into the extracellular milieu by many tumor cell types, which could be significant for tumor proliferation, migration, and angiogenesis (23). Thus, in tumor tissues, CTSD may degrade the extracellular matrix and basement membrane to promote migration. High expression of CTSD in tumors has been linked to rapid growth, poor differentiation, early migration, and poor prognosis. One study found that CTSD and MMP-7 expression was higher in pancreatic ductal adenocarcinoma patients than in healthy controls (24), and tumor cells overexpressing CTSD had high migration potential in a rat model (25). CTSD was found to stimulate the proliferation of MCF-7 cells (26) as well as breast, prostate, ovarian, and lung cancer cells (27-29).
In conclusion, we found that magnolol inhibited proliferation, migration, and induced apoptosis in four human pancreatic cancer cell lines by negatively regulating the expression of CTSD. These results suggest that magnolol is an effective natural alternative to currently used chemotherapeutic agents for the treatment of pancreatic cancer.
Acknowledgments
Funding: The present study was supported by the National Natural Science Foundation of China (grant No. 81302170), the Natural Science Foundation of Education Department of Sichuan Province (grant No. 16ZA0280), the Innovative Group Foundation of Education Department of Sichuan Province (grant No. 16TD0028), the Natural Science Foundation of Science and Technology Department of Sichuan Province (grant No. 2016JY0090), and the Natural Science Foundation of Chengdu Medical College (grant No. CYX12-029).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.08.36). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This article does not contain any studies with human participants or animals performed by any of the authors. Institutional ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet 2011;378:607-20. [Crossref] [PubMed]
- Cid-Arregui A, Juarez V. Perspectives in the treatment of pancreatic adenocarcinoma. World J Gastroenterol 2015;21:9297-316. [Crossref] [PubMed]
- Dimcevski G, Kotopoulis S, Bjånes T, et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J Control Release 2016;243:172-81. [Crossref] [PubMed]
- Caparello C, Meijer LL, Garajova I, et al. FOLFIRINOX and translational studies: Towards personalized therapy in pancreatic cancer. World J Gastroenterol 2016;22:6987-7005. [Crossref] [PubMed]
- Cheng YC, Hueng DY, Huang HY, et al. Magnolol and honokiol exert a synergistic anti-tumor effect through autophagy and apoptosis in human glioblastomas. Oncotarget 2016;7:29116-30. [Crossref] [PubMed]
- Zhang X, Huang H, Chang H, et al. Magnolol reduces bleomycin-induced rodent lung fibrosis. Int J Clin Exp Med 2015;8:15450-7. [PubMed]
- Li M, Zhang F, Wang X, et al. Magnolol inhibits growth of gallbladder cancer cells through the p53 pathway. Cancer Sci 2015;106:1341-50. [Crossref] [PubMed]
- Chen MC, Chen YL, Lee CF, et al. Supplementation of Magnolol Attenuates Skeletal Muscle Atrophy in Bladder Cancer-Bearing Mice Undergoing Chemotherapy via Suppression of FoxO3 Activation and Induction of IGF-1. PLoS One 2015;10:e0143594 [Crossref] [PubMed]
- Yang L, Zhu L, Ge G, et al. Species-Associated Differences in the Inhibition of Propofol Glucuronidation by Magnolol. J Am Assoc Lab Anim Sci 2014;53:408-11. [PubMed]
- You Q, Li M, Jiao G. Magnolol induces apoptosis via activation of both mitochondrial and death receptor pathways in A375-S2 cells. Arch Pharm Res 2009;32:1789-94. [Crossref] [PubMed]
- Hwang ES, Park KK. Magnolol suppresses metastasis via inhibition of invasion, migration, and matrix metalloproteinase-2/-9 activities in PC-3 human prostate carcinoma cells. Biosci Biotechnol Biochem 2010;74:961-7. [Crossref] [PubMed]
- Park JB, Lee MS, Cha EY, et al. Magnolol-induced apoptosis in HCT-116 colon cancer cells is associated with the AMP-activated protein kinase signaling pathway. Biol Pharm Bull 2012;35:1614-20. [Crossref] [PubMed]
- Lin SY, Liu JD, Chang HC, et al. Magnolol suppresses proliferation of cultured human colon and liver cancer cells by inhibiting DNA synthesis and activating apoptosis. J Cell Biochem 2002;84:532-44. [Crossref] [PubMed]
- Zhong WB, Wang CY, Ho KJ, et al. Magnolol induces apoptosis in human leukemia cells via cytochrome c release and Caspase activation. Anticancer Drugs 2003;14:211-7. [Crossref] [PubMed]
- Rasul A, Yu B, Khan M, et al. Magnolol, a natural compound, induces apoptosis of SGC-7901 human gastric adenocarcinoma cells via the mitochondrial and PI3K/Akt signaling pathways. Int J Oncol 2012;40:1153-61. [Crossref] [PubMed]
- Liu Y, Cao W, Zhang B, et al. The natural compound magnolol inhibits invasion and exhibits potential in human breast cancer therapy. Sci Rep 2013;3:3098. [Crossref] [PubMed]
- Bai X, Meng H, Ma L, et al. Inhibitory effects of evodiamine on human osteosarcoma cell proliferation and apoptosis. Oncol Lett 2015;9:801-5. [PubMed]
- Chen MC, Lee CF, Huang WH, et al. Magnolol suppresses hypoxia-induced angiogenesis via inhibition of HIF-1α/VEGF signaling pathway in human bladder cancer cells. Biochem Pharmacol 2013;85:1278-87. [Crossref] [PubMed]
- Kumar S, Guru SK, Pathania AS, et al. Autophagy triggered by Magnolol derivative negatively regulates angiogenesis. Cell Death Dis 2013;4:e889 [Crossref] [PubMed]
- Lee JH, Jung JY, Jang EJ, et al. Combination of honokiol and magnolol inhibits hepatic steatosis through AMPK-SREBP-1c pathway. Exp Biol Med (Maywood) 2015;240:508-18. [Crossref] [PubMed]
- Vidoni C, Follo C, Savino M, et al. The Role of Cathepsin D in the Pathogenesis of Human Neurodegenerative Disorders. Med Res Rev 2016;36:845-70. [Crossref] [PubMed]
- Stoka V, Turk V, Turk B. Lysosomal cathepsins and their regulation in aging and neurodegeneration. Ageing Research Reviews 2016;32:22-37. [Crossref] [PubMed]
- Garcia M, Platet N, Liaudet E, et al. Biological and clinical significance of cathepsin D in breast cancer metastasis. Stem Cells 1996;14:642-50. [Crossref] [PubMed]
- Park HD, Kang ES, Kim JW, et al. Serum CA19-9, cathepsin D, and matrix metalloproteinase-7 as a diagnostic panel for pancreatic ductal adenocarcinoma. Proteomics 2012;12:3590-7. [Crossref] [PubMed]
- Chen LC, Lee WS. P27/Kip1 is responsible for Magnolol-induced U373 apoptosis in vitro and in vivo. J Agric Food Chem 2013;61:2811-9. [Crossref] [PubMed]
- Wong SF, Seow HF, Lai LC. Effect of cathepsin D and prostate specific antigen on latent transforming growth factor-beta in breast cancer cell lines. Malays J Pathol 2003;25:129-34. [PubMed]
- Achour O, Ashraf Y, Bridiau N, et al. Alteration of cathepsin D trafficking induced by hypoxia and extracellular acidification in MCF-7 breast cancer cells. Biochimie 2016;121:123-30. [Crossref] [PubMed]
- Chen L, Li H, Liu W, et al. Olfactomedin 4 suppresses prostate cancer cell growth and metastasis via negative interaction with cathepsin D and SDF-1. Carcinogenesis 2011;32:986-94. [Crossref] [PubMed]
- Chai Y, Wu W, Zhou C, et al. The potential prognostic value of cathepsin D protein in serous ovarian cancer. Arch Gynecol Obstet 2012;286:465-71. [Crossref] [PubMed]


