
Preliminary study of the treatment effect of apatinib mesylate upon malignant ascites and its safety
Introduction
Ascites is a common symptom among advanced malignant tumor patients. They often suffer from abdominal distension, oliguria and poor appetite, and their living quality may be severely affected. At present, the major treatment method for malignant ascites is drainage, which is also combined with abdominal cavity perfusion. However, the overall treatment effect is modest, and patients can survive for only 2 to 6 months after treatment (1). Research has shown that vascular endothelial growth factor (VEGF) expression in malignant ascites is very high (2-5). This finding served as a theoretical basis for the application of anti-angiogenic drugs to treat malignant ascites in the current study.
Apatinib, a small molecule VEGF-2 tyrosine kinase inhibitor (TKI), is a first-generation oral anti-angiogenesis drug approved in the People’s Republic of China for use as second line of treatment for advanced gastric cancer. Previous research showed that, as small molecule TKIs, apatinib mesylate tablets mainly act on VEGFR as well as its downstream pathway that participates in controlling tumor angiogenesis. Their effectiveness against gastric, breast, and lung cancer has been validated in several lines of research (6-14). It was discovered in our clinical practice that malignant tumour patients with ascites had well-controlled ascites after taking apatinib orally. The current study was motivated by this observation. The application of apatinib in treating malignant ascites has not been reported so far. This paper aimed to explore the treatment effect of apatinib mesylate on malignant ascites and its safety.
Methods
Clinical data
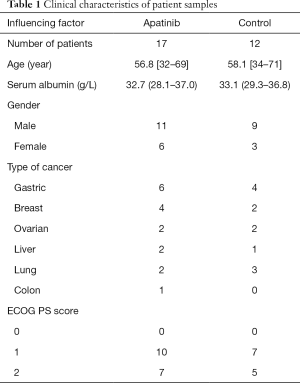
Data on 42 cancer patients who suffered from malignant ascites and were treated in the Oncology Department of Binzhou Medical College Affiliated Hospital from June 2015 to June 2016 were collected. All patients were cases with first-line treatment failure. The PS score was ≤2, and it was predicted that their survival time was 3 months or longer. In the course of follow-up treatment, 13 patients were felled off. The remaining 29 patients were divided into two groups by randomized block design: one group was treated with oral apatinib and abdominal cavity perfusion of cis-platinum (apatinib group, 17 patients), and another group was treated only through abdominal cavity perfusion of cis-platinum (control group, 12 patients). Clinical characteristics of patient samples in both groups were comparable. The study was approved by the Ethics Committee of the Binzhou Medical College Affiliated Hospital, and the patients selected for the study signed the consent form. Their clinical characteristics are shown in Table 1.
Full table
Reagents and medicines
VEGF enzyme-linked immunosorbent assay (ELISA) Kit was purchased from USCN. ELIASA 550 was purchased from BIO-RAD. cis-platinum was purchased from Qilu Pharmaceutical Factory. Apatinib mesylate was purchased from Jiangsu Renghui Medicine Co. Ltd. (Lianyungang, China).
Ascites VEGF detection
About 10 mL of ascites was collected and placed into a centrifugal tube, which was then centrifuged at 2,000 r/min for 10 min. The supernatant was placed in EP tubes and operated following the instruction on the ELISA kit.
Therapeutic method
After ultrasonic localization, all patients were placed with single-cavity drainage tube after abdominal puncture to drain as much ascite as possible. The 17 patients in the apatinib group were treated with oral apatinib mesylate and abdominal cavity perfusion of 60 mg/m2cis-platinum (combined group). Three weeks was deemed as a cycle. Patients in the control group were treated with abdominal cavity perfusion of cis-platinum only, and the perfusion dose and time interval were the same as those of the combined group. Patients in both groups were observed continuously for at least two cycles.
Evaluation criterion
Peritoneal effusion was measured by color Doppler ultrasound, and the results were evaluated by two radiologists (with over 5 years of work experience), with the average value of measurements taken as the final result. The WHO Evaluation Criterion for Treatment Effects upon Unmeasurable Focus was taken as the evaluation standard, which was composed of complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). The response rate (RR) was calculated based on CR + PR. Adverse events were evaluated according to Common Terminology Criteria for Adverse Events (CTCAE); it was composed of 1–5. The quality of life (QOL) was evaluated based on the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 (V3.0). It was conducted before treatment and after the second cycle. EORTC QLQ-C30 was composed of 30 items, including 5 function scales (somatic, role, cognitive, emotional and social functions), 3 symptom scales (fatigue, nausea/vomiting and pain), 5 single measurement items and 1 overall QOL scale (15-17). The subjects filled out questionnaires by themselves after they were given an introduction. Raw data of each scale was converted into a 0–100 scale based on the instruction of EORTC QLQ-C30. The standard score of each item was calculated. The higher scores of the function items and the overall health condition indicated that the patients had better physical condition, whereas the higher scores of the symptom items signified that the patients suffered more severe symptoms.
Statistical analysis
SPSS 16.0 was used for statistical analysis. Analysis of variance (ANOVA) was conducted for comparison of measurement data between both groups, and chi-square test was conducted for enumeration data. P<0.05 was deemed statistically significant.
Results
Short-term treatment effect
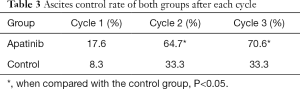
The apatinib group had 6 gastric cancer patients, 4 breast cancer patients, 2 ovarian cancer patients, 2 liver cancer patients, 2 non-small cell lung cancer patients and 1 colon cancer patient. On the other hand, the control group had 4 gastric cancer patients, 3 non-small cell lung cancer patients, 2 breast cancer patients, 2 ovarian cancer patients and 1 liver cancer patient. All patients, aged 32 to 71 years old, were diagnosed through ascites exfoliative cytologic examination to check if they were suffering from malignant ascites. All 29 patients completed the treatment, and the objective RR and safety were evaluated. A total of 5 patients obtained CR, 11 obtained PR, 13 obtained PD + SD and RR was 55.2%. The overall response rate of the apatinib group was 70.6% (12/17), whereas that of the control group was 33.3% (4/12). Statistical difference was found between both groups (x2 =3.948, P=0.047; see Table 2). Further analysis was conducted to evaluate control of ascites, and the results indicated that the ascite control rate in the apatinib group was 17.6% (3/17), whereas that in the control group was 8.3% (1/12) after one cycle. No statistically significant difference was found between both groups (x2 =0.513, P=0.474). After two cycles, the ascite control rate of the apatinib group was 64.7% (11/17), whereas that of the control group was 33.3% (4/12). A statistically significant difference was found between both groups (x2 =4.630, P=0.031), as shown in Table 3. The results indicated that the effects of treatment on the malignant ascites in the apatinib group became obvious starting the second or third week.

Full table
Full table
Comparison of VEGF level between both groups before and after treatment
The VEGF level of the apatinib group before treatment was 2,071.33±289.47 pg/mL, whereas the VEGF level of the control group before treatment was 2,398.19±333.20 pg/mL. No statistical difference was found between these groups (t =0.092, P=0.921). The VEGF level of the apatinib group after treatment was 586.10±129.46 pg/mL, which was significantly lower than the level before treatment 2,071.33±289.47 pg/mL (t =4.581, P<0.01) and also lower than the VEGF level of the control group after treatment 962.47±150.35 pg/mL. A statistically significant difference was found between these groups (t =2.173, P=0.038). Stratification analysis of the relationship between the initial ascites VEGF level and treatment effect showed that the VEGF level of the SD + PD group was significantly lower than that of the CR group (t =4.530, P<0.01) in the apatinib group. On the other hand, the VEGF level of the SD + PD group showed no statistically significant difference compared to that of the CR group (t =1.011, P=0.708) in the control group.
QOL
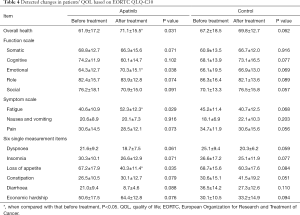
The results showed that in the apatinib group, no statistical difference was found in the function items (P>0.05) other than emotion (P<0.05) between before and after treatment. The overall health condition was improved after treatment (P<0.05). Evaluations of symptoms revealed a remarkable improvement in appetite (P<0.05), while fatigue intensified after treatment (P<0.05). Other symptoms, e.g., pain, showed some decrease but without statistically significant difference (P>0.05). In the control group, no statistically significant difference was found in any of the items (P>0.05) (see Table 4).
Full table
Safety
In this study, all the adverse effects (AEs) related to apatinib mesylate were of grades 1 to 3, and no medicine-related grade 4 AEs or serious adverse events took place. The most common AE was fatigue, which was found in 12 patients. This was followed by hypertension, found in 7 patients. Grade 3 AE occurred in 7 person-times, including fatigue, hand-foot-skin reaction, hypertension, proteinuria and sore throat. Among them, the incidence of fatigue and hypertension was 70.6% and 41.2%, respectively, whereas the incidence of hand-foot-skin reaction and proteinuria was 17.6%. In the apatinib group, 4 patients suffered from intestinal obstruction, with 2 patients showing complete intestinal obstruction. No one in the control group suffered from intestinal obstruction (Table 5).

Full table
Discussion
Previous studies have shown that VEGF could facilitate neovascularization and promote the increase of vascular permeability in tumor by exerting biological effects. VEGF is a key factor that drives the tumor cells to infiltrate and transfer to the abdominal cavity to form ascites (18,19). The VEGF content in ascites is high, and anti-angiogenic drugs, e.g., bevacizumab, and recombinant human endostatin, can control the formation of ascites by inhibiting VEGF, implying that anti-VEGF therapy is a potential therapeutic approach (2,20-23). However, its efficacy needs to be further improved. Additionally, since this approach has a high treatment cost, its clinical application is limited. Seventeen patients who took apatinib mesylate orally in combination with abdominal cavity perfusion of cis-platinum were enrolled and compared with the control group, which was composed of the patients who were treated with abdominal cavity perfusion of cis-platinum only. The results showed that the combined group’s short-term effect was good, and the RR was 70.6%, which is apparently higher than that of the control group. Further stratification analysis showed that after apatinib mesylate treatment, the high VEGF expression group had achieved a striking effect. The VEGF level showed remarkable decline after treatment (P<0.05), implying that the initial high level of ascites VEGF may be a good predictor of reaction to apatinib treatment. The emotional health as well as overall health condition and appetite of the apatinib mesylate group showed notable improvements in comparison with those of the control group. The difference between the two groups was statistically significant (P<0.05). However, the incidence of fatigue was greater in the treatment group compared to the control group, which may be attributed to the AEs of apatinib mesylate.
Common AEs, including fatigue, hypertension, proteinuria, neutropenia, thrombocytopenia, sore throat, hyponatremia and intestinal obstruction, were often found in the patients of the apatinib group during the follow-up period. The incidence of grades 3–4 AEs was lower than that reported in the literature (24-27). It may be related to the low dose adopted by patients in the group. It is notable that four intestinal obstruction cases were found in the apatinib group during the follow-up period, but none were found in the control group. Our results suggest that apatinib mesylate can control neovascularization and improve vascular permeability and accordingly allow the ascites to be controlled within the short term. In addition, apatinib mesylate results in the deposit of fibrinogen of ascites on the surface of the intestinal canal and aggravates intestinal adhesion. Whether the incidence of intestinal obstruction could be reduced by avoiding drugs that may aggravate intestinal adhesion, e.g., cis-platinum, or by adjusting the dose of apatinib mesylate, remains to be confirmed in clinical studies.
In summary, the combination of apatinib mesylate and abdominal cavity perfusion of cis-platinum has high objective response rate in treating malignant ascites, and it is safe and effective. The level of VEGF in the abdominal cavity can be used to predict its efficacy. Intestinal obstruction caused by the therapy requires close clinical attention, and physicians should try to avoid using drugs that may aggravate intestinal adhesion, e.g., cis-platinum. Since only a few cases were enrolled in this study, the treatment effect and safety of the therapy needs to be further verified in larger patient samples.
Acknowledgments
Funding: This work is supported by the Natural Science Foundation of Shandong Province (ZR2016HM41), Shandong Postdoctoral Innovation Fund [201601006], Changzhou High-Level Medical Talents Training Project (2016CZBJ016), the Medicine and Health and Scientific Development Programme of Shandong Province (2015WSB30011), and Scientific Development Programme of Binzhou Medical University (BY2014KJ45).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.08.24). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Binzhou Medical College Affiliated Hospital (No. 2017-010-01), and the patients selected for the study signed the consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Takahara N, Isayama H, Nakai Y, et al. Intravenous and intraperitoneal paclitaxel with S-1 for treatment of refractory pancreatic cancer with malignant ascites. Invest New Drugs 2016;34:636-42. [Crossref] [PubMed]
- Jones JM, Hardy JR, Munster DJ, et al. A pilot study of intraperitoneal bevacizumab for the palliation of malignant ascites. Asia Pac J Clin Oncol 2017;13:261-2. [Crossref] [PubMed]
- Bekes I, Friedl TW, Köhler T, et al. Does VEGF facilitate local tumor growth and spread into the abdominal cavity by suppressing endothelial cell adhesion, thus increasing vascular peritoneal permeability followed by ascites production in ovarian cancer? Mol Cancer 2016;15:13. [Crossref] [PubMed]
- Akutagawa N, Nishikawa A, Iwasaki M, et al. Expression of vascular endothelial growth factor and E-cadherin in human ovarian cancer: association with ascites fluid accumulation and peritoneal dissemination in mouse ascites model. Jpn J Cancer Res 2002;93:644-51. [Crossref] [PubMed]
- Nascimento IL, Barbosa HS, Schaer RE, et al. Evaluating angiogenic cytokines VEGF, basic FGF and TGF- β1 as tumor markers in ascites. J Clin Oncol 2005;23:9627. [Crossref]
- Huang L, Wei Y, Shen S, et al. Therapeutic effect of apatinib on overall survival is mediated by prolonged progression-free survival in advanced gastric cancer patients. Oncotarget 2017;8:29346-54. [PubMed]
- Aoyama T, Yoshikawa T. Apatinib - new third-line option for refractory gastric or GEJ cancer. Nat Rev Clin Oncol 2016;13:268-70. [Crossref] [PubMed]
- Peng S, Zhang Y, Peng H, et al. Intracellular autocrine VEGF signaling promotes EBDC cell proliferation, which can be inhibited by Apatinib. Cancer Lett 2016;373:193-202. [Crossref] [PubMed]
- Zhou N, Liu CM, Hou HL, et al. Response to apatinib in chemotherapy-failed advanced spindle cell breast carcinoma. Oncotarget 2016;7:72373-9. [PubMed]
- Ding L, Li QJ, You KY, et al. The Use of Apatinib in Treating Nonsmall-Cell Lung Cancer: Case Report and Review of Literature. Medicine (Baltimore) 2016;95:e3598 [Crossref] [PubMed]
- Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci 2011;102:1374-80. [Crossref] [PubMed]
- Ding J, Chen X, Gao Z, et al. Metabolism and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitorapatinib in humans. Drug. Metab Dispos 2013;41:1195-210. [Crossref] [PubMed]
- Fan M, Zhang J, Wang Z, et al. Phosphorylated VEGFR2 and hypertension: potential biomarkers to indicate VEGF-dependency of advanced breast cancer in anti-angiogenic therapy. Breast Cancer Res Treat 2014;143:141-51. [Crossref] [PubMed]
- Fontanella C, Ongaro E, Bolzonello S, et al. Clinical advances in the development of novel VEGFR2 inhibitors. Ann Transl Med 2014;2:123. [PubMed]
- Fayers PM, Aaronson NK, Bjordal K, et al. EORTC QLQ-C30 scoring manual. Brussels: EORTC Study Group on Quality of Life, 1995.
- Aaronson NK, Cull A, Kassa S, et al. The European Organization for Research and Treatment of Cancer (EORTC) modular approach to quality of life assessment in oncology. Int J Ment Health 1994;23:75-96. [Crossref]
- Olschewski M, Schulgen G, Schumacher M, et al. Quality of life assessment in clinical cancer research. British Journal of Cancer. 1994;70:1-5. [Crossref] [PubMed]
- Ferrara N, Gerber HP. The role of vascular endothelial growth factor in angiogenesi. Acta Haematol 2001;106:148-56. [Crossref] [PubMed]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669-76. [Crossref] [PubMed]
- Jordan K, Luetkens T, Gog C, et al. Intraperitoneal bevacizumab for control of malignant ascites due to advanced-stage gastrointestinal cancers: A multicentre double-blind, placebo-controlled phase II study - AIO SUP-0108. Eur J Cancer 2016;63:127-34. [Crossref] [PubMed]
- Zhao WY, Chen DY, Chen JH, et al. Effects of intracavitary administration of Endostar combined with cisplatin in malignant pleural effusion andascites. Cell Biochem Biophys 2014;70:623-8. [Crossref] [PubMed]
- Cheng D, Liang B, Kong H. Clinical significance of vascular endothelial growth factor and endostatin levels in the differential diagnosis of malignant and benign ascites. Med Oncol 2012;29:1397-402. [Crossref] [PubMed]
- Wu Y, Zhao M, Yang L, et al. Inhibition of malignant ascites with endostatin adenoviral vector. Sichuan Da Xue Xue Bao Yi Xue Ban 2004;35:316-9. [PubMed]
- Li J, Zhao X, Chen L, et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer 2010;10:529. [Crossref] [PubMed]
- Lee HJ, Moon JY, Baek SW. Is Treatment-Emergent Toxicity a Biomarker of Efficacy of Apatinib in Gastric Cancer? J Clin Oncol 2016; [Epub ahead of print]. [PubMed]
- Zhang S. Problematic Analysis and Inadequate Toxicity Data in Phase III Apatinib Trial in Gastric Cancer. J Clin Oncol 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol 2016;34:1448-54. [Crossref] [PubMed]


