
Retrospectively analysis of the pathology and prognosis of 131 cases of adenocarcinoma of the esophagogastric junction (Siewert type II/III)
Introduction
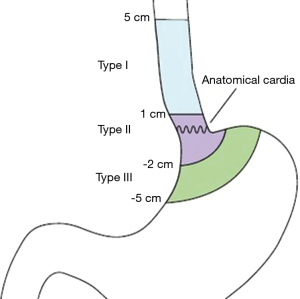
In recent years, although the total incidence of gastric cancer decreased gradually, the incidence of adenocarcinoma of the esophagogastric junction (AEG) has risen gradually, particularly in Europe and the United States. Unfortunately prognoses are still poorly made and the five-year survival rate is a mere 30% (1,2). The Surveillance, Epidemiology, and End Results (SEER) data system shows a 2.5-fold increase in the incidence of AEG in the last 35 years, and there are more male than female patients (3). The Siewert type classification of AEG, as defined by Siewert and Stein, came to be generally accepted by surgeons since its introduction in 1987 (4) and endorsement in 1998 by the International Gastric Cancer Association. Based on the distance of the tumour to the esophagogastric junction, there are three types of AEG: Siewert type I (between 1–5 cm above the junction), Siewert type III (between 2–5 cm below the junction) (5), and Siewert type II (in the immediate vicinity of the junction) (Figure 1).
Studies showed that the lymph node metastasis and biological behaviour of AEGs are obviously different from esophageal and gastric cancer (6). Also aspects of treatment and prognosis for AEG are distinct from esophageal and gastric cancer. At present, there are no specific treatment guidelines for this kind of disease and thus treatments are based on existing guidelines for gastric cancer and esophageal therapy. New research is currently primarily focused on lymph node metastasis, surgical approaches and surgical resection ways. This study collected 131 AEG cases (Siewert type II/III) from the Guangdong General Hospital between 2004 and 2012 to assess pathological features, and clinical prognosis factors; and to share our experience treating AEG patients.
Methods
Patients
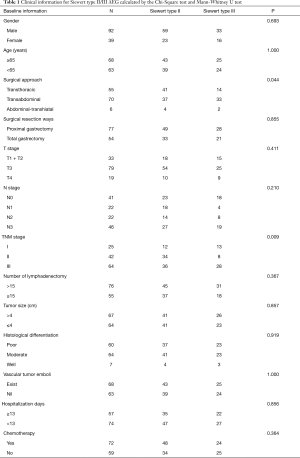
Clinical data and follow-up information (the last follow-up time is 2016-2010) of 131 AEG cases between 2004 and 2012 were collected from the Guangdong General Hospital (Table 1); 82 cases of Siewert type II and 49 cases of Siewert type III. The Siewert type was determined from either preoperative CT scans or upper gastrointestinal imaging obtained by radiologists, intraoperative surgical records (surgeons) or postoperative pathological specimens (pathologists). Neither of the patients had received preoperative chemoradiotherapy. Proximal or total gastrectomy (D1+/D2/D2+ lymphadenectomy according to the 4th Japanese Gastric Cancer Treatment guidelines) was performed on all patients using the transabdominal or transthoracic approach. Postoperative pathology confirmed the adenocarcinoma and TNM stages were determined according to the 7th edition of AJCC/UICC TNM staging system for gastric cancer.
Full table
Analysis data
Based on the literature, gender, age, the number of hospitalisation days, blood type, Siewert type, T stage, N stage, TNM stage, histological differentiation, tumour size, the number of lymphadenectomies, vascular tumour emboli, surgical resection ways, surgical approach, simultaneous organ resection, intraoperative blood loss and finally postoperative adjuvant chemotherapy were taken into account in the retrospective analysis.
Follow-up
Overall survival was calculated from the time of surgery until death or the last follow-up contact. Follow-up assessments in the form of outpatient visits or telephone interviews were carried out every 3–6 months for the first 2 years, every 6–12 months between years three and five post-surgery and then annually every year according to National Comprehensive Cancer Network guidelines. Follow-up information was updated until October 2016 (to meet the 3-year survival rate analysis). Incomplete follow-up information was often due to refusal of outpatient visits or change of telephone number and address.
Statistical analysis
Statistical analysis was performed using SPSS 19.0. Categorical data were compared by chi-square test tests or Fisher exact test. Survival curves were derived from Kaplan-Meier estimates and the curves were compared by log-rank test. Prognostic factors were identified by univariate analysis, and further examined by multivariate analysis. The multivariate analysis was performed with the Cox proportion hazards model. In our Cox proportional hazards model, P<0.05 was defined as the inclusion criteria. The P value less than 0.05 was considered statistical significance.
Results
General information
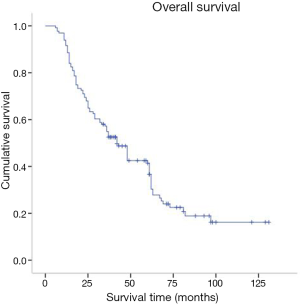
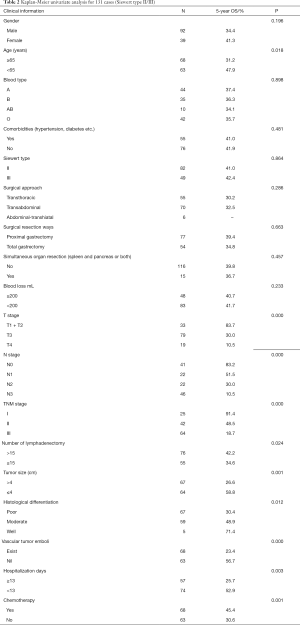
Preoperative and follow-up information was obtained from a total of 131 AEG patients in this study of which 12 cases were eventually dismissed due to a lack of follow-up information. By October 2016, 91 patients (69.4%) were reported deceased. Postoperative survival rates were 92.6%, 52.5% and 36.6% for years one, three and five respectively (Figure 2). Patients were between 24 and 86 years old at the time of diagnosis, the average age was 63.16, the median age was 65, and there were 68 patients older than 65. There were 82 Siewert type II and 49 Siewert type III patients, 92 patients were male and 39 females. There were 92 male patients and 39 female patients. Forty-four patients had comorbidities, often hypertension or diabetes mellitus; 76% of patients had progressive dysphagia, 48% had superior abdominal pains and posterior sternum pains, and 22% suffered from acid regurgitation and belching.
There were 55 cases with transthoracic, 70 with transabdominal, and six with a combined thoraco-abdominal operation. Surgical resection ways included proximal gastrectomy (77 cases) and total gastrectomy (54 cases), simultaneous organ resection (spleen, pancreas, or both) happened in 15 cases. The average intraoperative blood loss was 156.2 mL, the median blood loss was 200 mL. There were 48 cases where more than 200 mL blood was lost.
Tumour sizes ranged between 1.20 and 12.00 cm with an average tumour size of 4.62 cm and a median of 4.20 cm. There were 67 cases where the radius was larger than 4.00 cm. TNM stages of cases were: 25 with stage I, 42 in stage II and 64 with Stage III. In terms of T stage there were four cases with T1, 29 with T2, 79 with T3 and 19 with T4. The number of lymphadenectomies ranged from 0 to 39, with 18.13 the average and 18 the median. In 76 cases the number of lymphadenectomies was higher than 15. The average number of positive lymph nodes was 5.08 and the median was 3. In terms of N stage, there were 41 cases with N0, 22 with N1, 22 with N2 and 46 with N3. In as many as 60 cases the adenocarcinomas were histologically poorly differentiated; this includes seven cases with moderate to poor differentiation records. Another 64 cases reported moderate differentiation and 7 were well differentiated. Sixty-eight cases had vascular tumour emboli. On average patients spent 15.13 days in hospital. The median number of hospitalisation days was 13 and in 57 cases patients stayed longer than 13 days. There were 68 cases of adjuvant chemotherapy. Postoperative relapse and metastasis was reported in 23 of the cases and the average relapse time was 16.8 months.
Univariate analysis
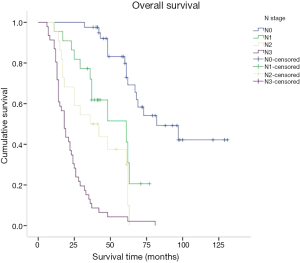
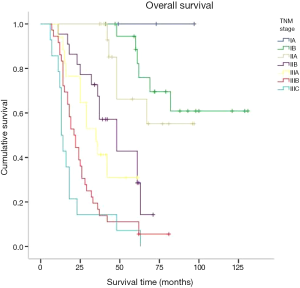
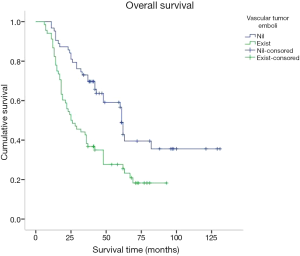
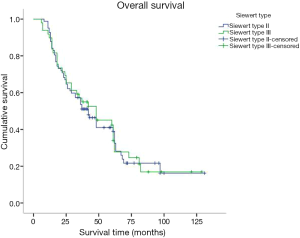
Kaplan-Meier was used for survival analysis and indicated that age, the number of hospitalisation days, T stage, N stage, TNM stage, the number of lymphadenectomies, histological differentiation, tumour size, vascular tumour emboli and adjuvant chemotherapy were all factors affecting the prognosis for the patients (P<0.05; Figures 3-5). Gender, blood type, Siewert type (Figure 6), surgical approach, surgical resection way, simultaneous organ resection and comorbidities on the other hand were statistically irrelevant for the prognosis (P>0.05) (Table 2).
Full table
Cox multivariate analysis
Factors contributing to the prognosis for AEG patients as per the univariate analysis were also included in a Cox multivariate survival analysis. These factors included age, T stage, N stage, TNM stage, the number of lymphadenectomies, histological differentiation, tumour size, vascular tumour emboli and adjuvant chemotherapy. Cox multivariate analysis suggested that both N stage and the presence of vascular tumour emboli were independent factors affecting AEG prognosis (Tables 3,4).
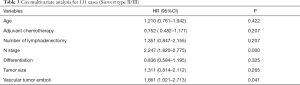
Full table
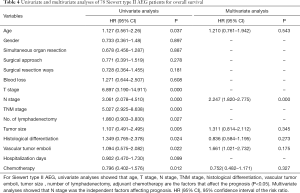
Full table
Discussion
AEG is a type of malignancy that bestrides the esophageal and gastric carcinoma (1,6). However, other researchers (7,8) have highlighted the differences between AEG and these two cancers in terms of biological behaviour and pathologic characteristics. Therefore AEG classification, diagnosis, management, and prognosis are still under scrutiny. North American and European researchers believe that AEG is similar in epidemiology, clinical features, local infiltration, lymph node metastasis and prognosis to Barrett’s esophagus, which itself is related to esophageal carcinoma. Barrett’s esophagus is a seven TMN stage esophageal carcinoma according to the American Joint Committee on Cancer (AJCC) (9). According to National Comprehensive Cancer Network guidelines, Siewert type I and II AEG are classified into the therapeutic range of esophageal carcinomas, and Siewert type III as a gastric cancer (10). In Asia, researchers believe that Siewert type II AEG is the same as gastric cancer, and thus we put Siewert type II and III AEG together for unified analysis and research (2,6,11).
In our study there were 92 cases in males and 39 cases in females, a ratio of 2.35. This is comparable to the gender ratio of 2.88 for AEG reported by the Sun Yat-sen University cancer centre in 2010 (12). Research showed that 28% of AEG in Europe was diagnosed in men and nearly half of the male gastric carcinomas were AEG (13). Further, AEG is often diagnosed in elders (11). This is also reflected in our study where the average age of patients was 63.16 years and in 68 cases patients were older than 65. The early stage of AEG cannot be distinguished from gastric carcinoma in terms of clinical symptoms. In this study, about 76% of patients were diagnosed with progressive dysphagia and poor prognosis comparatively. The ratio of Siewert type II to type III approximated four fifth, which is similar to a previous report—Huang et al. (14). Sixty-eight patients in our study underwent postoperative adjuvant chemotherapy. Many researchers (15,16) have testified that such treatment can improve the five year survival rate and decrease the chances of relapse. Indeed, our study confirmed that postoperative adjuvant chemotherapy affected patient’s prognosis for AEG. In addition, the prognosis for AEG is worse than for esophageal cancer and gastric carcinoma according to domestic and overseas reports (17-19) suggesting a 5-year survival rate of 22.6% to 50%. This is in agreement with the data presented here where 1-, 3- and 5-year survival rates were 91.6%, 52.5%, and 36.6% respectively. Kaplan-Meier single factor analysis showed a correlation between prognosis and the age and number of hospitalisation days. Although there was no correlation between gender and prognosis, our study suggested towards a longer survival time for females (48 months) than male patients (37 months). This has also been observed and reported elsewhere (13).
There is some consensus that surgical approach and resection way are key for AEG treatment (9). Siewert type I is classified in the lower part of esophageal cancer treatment guidelines. Therefore a transthoracic surgical approach and a mediastina lymph node dissection is often chosen by specialists. The Siewert type III on the other hand, is classified in the upper gastric cancer, which can be resected by total gastrostomy plus D2 lymph node dissection according to the 4th Japanese Gastric Cancer treatment guideline (17). Surgical approach and surgical resection way have been a controversial clinical issue for AEG treatment. Studies have shown that, for AEG, a different surgical approach (transthoracic approach or transabdominal approach) and surgical resection way (proximal gastrectomy, total gastrectomy) may affect lymph node dissection, overall prognosis and the number of complications (18-20). The Japanese Clinical Oncology Group presented data from 9,502 clinical trials (21), including 167 cases of AEG of which 95 were Siewert type II and 63 were Siewert type III, and show that the transabdominal approach had a survival advantage for AEG patients. Others (22) however believe that total gastrostomy would work better as it can reduce the need for lymph node dissection resulting in a better prognosis. Liu et al. (23) performed a meta-analysis on data from 10 studies with 2,481 patients in order to compare the two surgical resection ways for Siewert type II and type III, and found no statistical differences. Our study confirmed these data as the univariate analysis showed no correlation between the survival of patients and the surgical approach and surgical resection way. Our data comprised of 55 transthoracic and 70 transabdominal cases; and 77 proximal and 54 total gastrostomy cases. AEG lymph node metastasis targets the No. 1,2,3,5,7 lymph nodes (21). Irrespective of the surgical approach or the surgical resection way, No. 1,2,3,5,7 lymph nodes could all be successfully dissected and obtained the same prognosis. In agreement with Fujitani et al. (24), pathological parameters including T stage, N stage, TNM stage, histological differentiation, lymph node cleaning count and the presence of vascular tumour emboli all correlated with AEG prognosis. Further to lymph node metastasis being an important factor for AEG prognosis, our study included 41 cases in N0 stage, 22 in N1, 22 in N2 and 46 in N3 with significantly different 5-year survival rates of 72.9%, 41.2%, 28.9% and 7.2% respectively. Also significantly different were the 5-year survival rates of AEG patients with TNM stage I (81%, 25 cases), stage II (39.7%, 42 cases) and stage III (11.2%, 64 cases). Cox multi-factor survival analysis demonstrated that N stage and the presence of vascular tumour emboli are independent factors affecting the prognosis for AEG, which is in agreement with previous reports (2,25). In our study, 80.9% of cases were in progressive stages II or III. The survival rate of stage I AEG patients is obviously poor and thus for patients with AEG it is critical that early screening programs are introduced. By conveying the importance of early diagnosis and early management we hope to improve our country’s early diagnostic rate of AEG and thus improve prognosis (6).
This study was conducted to illustrate Siewert type II/III factors affecting the prognosis for AEG patients. This study has laid the foundation for future clinical managing and prognosis monitoring of the disease and stressed the importance of an early diagnosis. Limitations to this study such as incomplete laboratory test indicators (CEA CA19-9hemoglobin, etc.), deletion of immunohistochemical results (HER2) one site location, a small sample number and retrospective data have been acknowledged. To verify the results presented from this study future work needs to obtain prospective data from multiple locations and have a larger sample number.
Acknowledgments
Funding: This study is supported by Natural Science Foundation of Guangdong Province (2016A030310328).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.09.18). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards and written informed consent was obtained from all individual participants included in the study. Institutional ethical approval was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Deng JY, Liang H. Adenocarcinoma of esophagogastric junction. Chin J Cancer Res 2014;26:362-3. [PubMed]
- Hasegawa S, Yoshikawa T. Adenocarcinoma of the esophagogastric junction: incidence, characteristics, and treatment strategies. Gastric Cancer 2010;13:63-73. [Crossref] [PubMed]
- Buas MF, Vaughan TL. Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Semin Radiat Oncol 2013;23:3-9. [Crossref] [PubMed]
- Siewert JR. Adenocarcinoma of the esophago-gastric junction. Gastric Cancer 1999;2:87-8. [Crossref] [PubMed]
- Strong VE, D'amico TA, Kleinberg L, et al. Impact of the 7th Edition AJCC staging classification on the NCCN clinical practice guidelines in oncology for gastric and esophageal cancers. J Natl Compr Canc Netw 2013;11:60-6.
- Suh YS, Kong SH, Lee HJ, et al. Reply to the letter: "should adenocarcinoma of the esophagogastric junction be classified as gastric or esophageal cancer, or else as a distinct clinical entity? Ann Surg 2015;261:e67-8. [Crossref] [PubMed]
- Goodman KA. Esophagogastric junction carcinoma: introduction. Semin Radiat Oncol 2013;23:1-2. [Crossref] [PubMed]
- Odemis B, Cicek B, Zengin NI, et al. Barrett's esophagus and endoscopically assessed esophagogastric junction integrity in 1000 consecutive Turkish patients undergoing endoscopy: a prospective study. Dis Esophagus 2009;22:649-55. [Crossref] [PubMed]
- Siewert JR, Feith M. Adenocarcinoma of the esophagogastric junction: competition between Barrett and gastric cancer. J Am Coll Surg 2007;205:S49-53. [Crossref] [PubMed]
- Hosokawa Y, Kinoshita T, Konishi M, et al. Clinicopathological features and prognostic factors of adenocarcinoma of the esophagogastric junction according to Siewert classification: experiences at a single institution in Japan. Ann Surg Oncol 2012;19:677-83. [Crossref] [PubMed]
- Li B, Xiang J, Zhang Y, et al. Factors affecting hospital mortality in patients with esophagogastric anastomotic leak: a retrospective study. World J Surg 2016;40:1152-7. [Crossref] [PubMed]
- Zheng B, Chen YB, Hu Y, et al. Trend analysis for clinical characteristics and prognosis of adenocarcinoma of cardia. Chin J Cancer 2010;29:94-7. [Crossref] [PubMed]
- Di Martino N, Izzo G, Cosenza A, et al. Surgical therapy of adenocarcinoma of the esophagogastric junction: analysis of prognostic factors. Hepatogastroenterology 2005;52:1110-5. [PubMed]
- Huang CM, Lv CB, Lin JX, et al. Laparoscopic-assisted versus open total gastrectomy for Siewert type II and III esophagogastric junction carcinoma: a propensity score-matched case-control study. Surg Endosc 2016; [Epub ahead of print]. [PubMed]
- Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21. [Crossref] [PubMed]
- Zhao Y, Dai Z, Min W, et al. Perioperative versus preoperative chemotherapy with surgery in patients with resectable squamous cell carcinoma of esophagus: a phase III randomized trial. J Thorac Oncol 2015;10:1349-56. [Crossref] [PubMed]
- Zhang YF, Shi J, Yu HP, et al. Factors predicting survival in patients with proximal gastric carcinoma involving the esophagus. World J Gastroenterol 2012;18:3602-9. [Crossref] [PubMed]
- Feith M, Stein HJ, Siewert JR. Adenocarcinoma of the esophagogastric junction: surgical therapy based on 1602 consecutive resected patients. Surg Oncol Clin N Am 2006;15:751-64. [Crossref] [PubMed]
- Schuhmacher C, Novotny A, Feith M, et al. The new TNM classification of tumors of the esophagogastric junction. Surgical consequences. Chirurg 2012;83:23-30. [Crossref] [PubMed]
- Chen XZ, Zhang WH, Hu JK. Lymph node metastasis and lymphadenectomy of resectable adenocarcinoma of the esophagogastric junction. Chin J Cancer Res 2014;26:237-42. [PubMed]
- Sasako M, Sano T, Yamamoto S, et al. Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: a randomised controlled trial. Lancet Oncol 2006;7:644-51. [Crossref] [PubMed]
- Kakeji Y, Yamamoto M, Ito S, et al. Lymph node metastasis from cancer of the esophagogastric junction, and determination of the appropriate nodal dissection. Surg Today 2012;42:351-8. [Crossref] [PubMed]
- Liu Y, Han G, Wang G, et al. Proximal gastrectomy versus total gastrectomy for adenocarcinoma of esophagogastric junction: a Meta-analysis. Zhonghua Wei Chang Wai Ke Za Zhi 2014;17:373-7. [PubMed]
- Fujitani K, Miyashiro I, Mikata S, et al. Pattern of abdominal nodal spread and optimal abdominal lymphadenectomy for advanced Siewert type II adenocarcinoma of the cardia: results of a multicenter study. Gastric Cancer 2013;16:301-8. [Crossref] [PubMed]
- Liu Y, Du CX, Zhang HG. Analysis of the prognosis of 111 patients with gastric cancer or adenocarcinoma of the esophagogastric junction combined with pleural or abdominal effusion. Zhonghua Zhong Liu Za Zhi 2013;35:693-7. [PubMed]


