
Outcomes in BRCA mutation carriers: evaluation of current data for optimal clinical care
BRCA1 and BRCA2 are genes responsible for homologous recombination and repair of double-strand DNA breaks (1). Mutations in either gene increase the risk of malignancy and are responsible for the hereditary breast and ovarian cancer syndrome (HBOC). The lifetime incidence of breast cancer in those harboring BRCA1 or BRCA2 mutations has been estimated to be greater than 80% (2,3). The biology of BRCA1/2 mutation associated cancers is unique. BRCA1 mutated tumors in particular are often estrogen receptor (ER) and HER2 negative and express a phenotype of a basal-type breast cancer (4). They are typically higher-grade tumors, with more metastatic potential and poorer outcomes (5). There has also been interest in the potential susceptibility of BRCA-mutated cancers to therapeutic agents, such as platinum-derived chemotherapy and PARP inhibitors, that take advantage of the defect in homologous DNA repair, so-called “synthetic lethality” (6).
Despite an understanding of the incidence and biology of these BRCA-associated cancers, there has been no clear evidence on the impact of these mutations on breast cancer-specific survival compared to non-carriers. Prior studies have been conflicting and plagued by small numbers, selection, and survivor bias (7,8). The latter is often secondary to including women who were able to agree to blood-based genetic testing selected for those who did not suffer from early recurrence and mortality. Retrospective tissue based testing has been seen as a way to avoid this pitfall, but small numbers and methodology often not accounting for risk-reducing intervention have prevented clear insight.
In their publication in the Journal of the National Cancer Institute, Schmidt, van den Broek et al. analyzed a cohort of 6,478 women younger than 50 years of age with breast cancer to assess long-term survival differences between BRCA1/2 mutation carriers and non-carriers (9). This was an unselected cohort of women from ten Dutch hospitals. To avoid survivor bias, the investigators determined germline BRCA1/2 mutation status using formalin-fixed paraffin-embedded non-tumor tissue for most patients. After excluding those with synchronous bilateral cancers, metastatic disease at or within 3 months of diagnosis, and without adequate DNA for analysis; 6,304 patients were suitable for overall survival (OS) analysis. Fewer patients had sufficient clinical data for assessment of breast cancer-specific survival and disease and metastasis-free survival, 3,515 and 3,440 respectively. Median follow-up was an impressive 14 years. BRCA1 and BRCA2 mutation carriers made up 3.2% and 1.2% of the patients in this large cohort. The absolute difference in OS was about 10% for BRCA1 (61.4%) BRCA2 (60.95%) carriers compared to non-carriers (70.4%).
The 210 BRCA1 carriers were more likely to develop ER-negative and triple-negative disease. In fact, 62.6% of BRCA1 carriers had triple-negative disease as opposed to only 18.3% of non-carriers. Perhaps unsurprisingly then, BRCA1 carriers had a statistically significant worse OS with a hazard ratio (HR) for death of 1.28 (95% CI =1.05 to 1.57, P=0.01) compared to non-carriers, as described in Table 1. This difference appeared to be driven by mortality within the first 5 years of diagnosis, with a HR of 1.86 (95% CI =1.43 to 2.41, P≤0.001) during this period. OS variance was mitigated when adjusted for known prognostic indicators such as ER-receptor status, nodal status and treatment strategies; the adjusted HR being 1.2 (95% CI =0.97 to 1.47, P=0.09) for all follow-up and 1.4 (95% CI =1.07 to 1.84, P=0.02) for the first 5 years of follow-up. The investigators were unable to demonstrate a statistically significant change in breast cancer-specific survival in the adjusted group, even within the first 5 years of diagnosis. BRCA1 was associated with increased risk of ovarian cancer, which was as expected a risk for death in BRCA1 carriers and non-carriers. In fact, analysis of OS only including BRCA1 patients prior to incidence of ovarian cancer largely negated the disparity in OS with a HR of 1.10 (95% CI =0.88 to 1.36, P=0.42). Taken together, this suggests the differential OS was driven largely by expected clinical characteristics such as more triple-negative disease and an increased risk of mortality from second ovarian cancers. Another notable finding is that not receiving adjuvant chemotherapy, even when adjusted for clinical and other treatment parameters, appeared to be a greater risk for mortality in BRCA1 carriers compared to non-carriers. The adjusted HR was 1.54 (95% CI =1.08 to 2.19, P=0.02) in the BRCA1 mutated group compared to control.

Full table
Analysis of BRCA2 carriers is more limited given the small cohort of 75 patients. Results are summarized in Table 2. BRCA2 carriers had a non-significant difference in OS regardless of clinical and pathologic factors with an unadjusted HR of 1.26 (95% CI =0.91 to 1.73, P=0.16) and adjusted HR of 1.06 (95% CI =0.77 to 1.47, P=0.71). They tended to be higher grade and involve lymph nodes. They had an inverted pattern of mortality compared to BRCA1 carriers, with worse survival beyond 5 years of follow-up, as evidenced by a HR of 1.56 (95% CI =1.06 to 2.28, P=0.02). Adjusted for clinic-pathological factors, the HR for death after 5 years of follow-up was 1.47 (95% CI =1.00 to 2.17, P=0.05). The difference in OS including all years of follow-up was not statistically significant.
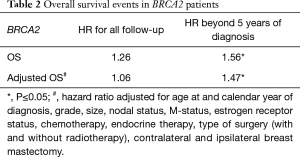
Full table
This retrospective analysis has a number of strengths. BRCA1/2 testing was done on fixed non-tumor for the majority of patients. Moreover, 4,642 of 6,478 of the specimens were collected before 1995 meaning patients and clinicians were unlikely to be aware of BRCA1/2 mutation status at time of treatment. However, the authors do note that many of these patients would go on to have eventual testing and likely increased screening, perhaps accounting for no difference in survival for those with second primary breast cancers. Results may have been diluted by the accidental inclusion of carriers in the inappropriate group as the authors state the BRCA1/2 mutations tested account for about 61% of BRCA1/2 mutations prevalent in the Netherlands. Another important observation was the influence of secondary cancers on outcome, as many prior studies had failed to address the competing influence of ovarian cancer mortality. The overarching result that BRCA-related breast cancers have similar outcomes to other, sporadic cancers with similar clinical and pathologic features is compatible with results from analysis of an Israeli cohort. Rennert et al. investigated outcomes for women diagnosed with breast cancer from 1987 to 1988 in the Israel National Cancer Registry (10). They used fixed tissue for BRCA1/2 testing and their cohort was not selected based on age or other clinical features. They found no difference in OS between carriers and non-carriers. However, they similarly observed earlier mortality among BRCA1 carriers. Eighty-eight percent of deaths in BRCA1 carriers occurred before 5 years of follow-up. BRCA2 carriers had no statistically significant difference from non-carriers in terms of OS in either study. Limitations of this study include the majority of patients having unknown hormone receptor status. Schmidt and van den Broek et al. were able to obtain receptor status on all but 25.1% of patients. Their results are also compatible with their prior systematic review demonstrating only moderate evidence for a link to worsened OS in BRCA carriers when accounting for other risk factors (8).
As the authors have previously noted, the association with a lack of adjuvant therapy and worse survival in BRCA1 patients is of interest. The results discussed here appear to confirm this concern. It may suggest a differential benefit to chemotherapy treatment in BRCA1/2 carriers that normalizes risk in those treated with adjuvant chemotherapy. With the caveat that the study enrolled patients from 1970 to 2003, and few were likely to receive contemporary adjuvant chemotherapeutic regimens, the findings again raise the question of biological susceptibility enhancing the effect of chemotherapy in BRCA1/2 deficiency. The pattern of worsened survival in the first 5 years after treatment of BRCA1 carriers raises the question of earlier and more aggressive relapse in this group, even when accounting for traditional prognostic factors. The aforementioned Israeli registry study also demonstrated a statistically significant worse survival for BRCA1 carriers who did not receive chemotherapy compared to non-carriers who did not receive chemotherapy with an adjusted HR of 1.59 (95% CI =1.01 to 2.50, P=0.04). This finding is also congruent with findings from a cohort of North American Ashkenazi Jewish women, also carried out retrospectively on fixed tissue to avoid selection bias, that showed BRCA1 status predicted breast cancer mortality only among women who did not receive chemotherapy (HR 4.8, 95% CI: 2.0 to 11.7; P=0.001) (11).
Based on the studies presented to date it seems that BRCA1/2 mutation carriers have similar survival patterns compared with non-carriers when controlling for type of cancer, treatment, age at diagnosis, and secondary ovarian cancers. BRCA1 mutation carriers are more likely to develop TNBC and to have improved responses to chemotherapy (12,13). BRCA1/2 mutation carriers with ER positive breast cancers have higher Oncotype Dx scores, providing again evidence of more aggressive disease amenable to chemotherapy (14). These findings help us clinically in focusing on prevention of breast cancer in BRCA1/2 mutation carriers, aggressive treatment of BRCA-related breast cancer and prevention of subsequent malignancies such as ovarian cancer and contralateral breast cancer.
Acknowledgments
Funding: Dolores Knes Fund (VG Kaklamani).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor San-Gang Wu (Department of Radiation Oncology, Xiamen Cancer Center, The First Affiliated Hospital of Xiamen University, Xiamen, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.09.49). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tutt A, Ashworth A. The relationship between the roles of BRCA genes in DNA repair and cancer predisposition. Trends Mol Med 2002;8:571-6. [Crossref] [PubMed]
- Ford D, Easton DF, Bishop DT, et al. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet 1994;343:692-5. [Crossref] [PubMed]
- Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1998;62:676-89. [Crossref] [PubMed]
- Foulkes WD, Stefansson IM, Chappuis PO, et al. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst 2003;95:1482-5. [Crossref] [PubMed]
- Bauer KR, Brown M, Cress RD, et al. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer 2007;109:1721-8. [Crossref] [PubMed]
- Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005;434:917-21. [Crossref] [PubMed]
- Cortesi L, Masini C, Cirilli C, et al. Favourable ten-year overall survival in a Caucasian population with high probability of hereditary breast cancer. BMC Cancer 2010;10:90. [Crossref] [PubMed]
- van den Broek AJ, Schmidt MK, van 't Veer LJ, et al. Worse breast cancer prognosis of BRCA1/BRCA2 mutation carriers: what's the evidence? A systematic review with meta-analysis. PLoS One 2015;10:e0120189 [Crossref] [PubMed]
- Schmidt MK, van den Broek AJ, Tollenaar RA, et al. Breast Cancer Survival of BRCA1/BRCA2 Mutation Carriers in a Hospital-Based Cohort of Young Women. J Natl Cancer Inst 2017;109: [Crossref] [PubMed]
- Rennert G, Bisland-Naggan S, Barnett-Griness O, et al. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med 2007;357:115-23. [Crossref] [PubMed]
- Robson ME, Chappuis PO, Satagopan J, et al. A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. Breast Cancer Res 2004;6:R8-R17. [Crossref] [PubMed]
- Byrski T, Huzarski T, Dent R, et al. Response to neoadjuvant therapy with cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat 2009;115:359-63. [Crossref] [PubMed]
- Tassone P, Tagliaferri P, Perricelli A, et al. BRCA1 expression modulates chemosensitivity of BRCA1-defective HCC1937 human breast cancer cells. Br J Cancer 2003;88:1285-91. [Crossref] [PubMed]
- Lewin R, Sulkes A, Shochat T, et al. Oncotype-DX recurrence score distribution in breast cancer patients with BRCA1/2 mutations. Breast Cancer Res Treat 2016;157:511-6. [Crossref] [PubMed]


