
A study of EGFR wild-type non-small cell lung cancer ALK genetic mutation
Introduction
Lung cancer is one of the most common malignancies in the world. the morbidity and mortality are the first among all cancers (1). About 80–85% of lung cancers are non-small cell lung cancer (NSCLC) patients. A total of 70% of patients have been diagnosed at the advanced stage, those lose the chance of surgical treatment. The first-line platinum containing double dose chemotherapy has limited efficacy. Targeted therapies for genes such as EGFR, ALK and KRAS have become the hotspot of attention and study in lung cancer treatment for the last 10 years. To clarify the positive rate and clinicopathological features of EML4-ALK, the relationship between EGFR mutations in NSCLC patients, we selected 300 tissue samples which come from NSCLC operation or biopsy. Using automatic immunohistochemistry to detect the expression of ALK protein, to provide reference for clinical diagnosis and treatment.
Methods
General information
Three hundred cases of NSCLC diagnosed were collected by Affiliated Tumor Hospital of Guangxi Medical University in October 2011–December 2014, including 178 males (178/300, 59.33%) and 122 females (122/300, 40.67%), minimum age 33 years, maximum age 87 years, average age 58.5 years; adenocarcinoma 239 cases (239/300, 79.67%), squamous carcinoma 36 cases (36/300, 12%), mixed carcinoma 10 cases (10/300, 3.33%), other carcinoma 15 cases (15/300, 5%). Han 238 cases (79.33%), Zhuang 56 cases (18.67%), other nationalities 6 cases (2%).
Pre-processed of samples
Fresh tumor tissue was fixed with 4% neutral formaldehyde after 1 h in vitro, the fixed liquid volume was 10 times of tissue volume, the fixation time was 6–48 h, paraffin imbedded, section.
EGFR gene mutation in NSCLC was detected by ARMS (amplification refractory mutation system)
- Examined HE sections of the samples were cut by the tumor region selected by the pathologist for gene testing;
- DNA extraction: paraffin block in selected area was cut 10–15 pieces of paraffin sections, thick 5 mm, dewaxed by xylene, soaked by anhydrous ethanol, washed by water, dried. Then add lysis liquid and 55% Proteinase k, stay overnight in the water bath box. centrifugation supernatant solution, extract DNA according to the instructions;
- Detection of mutations of EGFR gene: apply reagent kit which developed by Xiamen Aide Biological Medicine Science and Technology Co. Ltd. for detecting human EGFR gene mutation (PCR, ARIVIS). Detecting of EGFR gene (exon 18–21) including 29 species of mutations, specific steps according to instructions of reagent kit.
Tissue chip making
Observing H&E sections of the tumor tissue samples under microscope. Taking to the diameter tissue which standard is 1.5 mm. Preparing 60 lattices which spacing is 2 mm at each tissue chip.
Using automatic immunohistochemistry to detect the expression of ALK protein
Materials
All of immunohistochemistry including anti ALK (D5F3) monoclonal antibody, OptiView DAB kit, enhanced amplification kit was produced by Roche/Ventana. Instrument of immunohistochemical staining used to dyeing were BenchMark XT produced by Roche/Ventana.
Tissue chip made by Pantomics Inc., slicing machine; tablet press 40 °C; baking machine 65 °C; marking machine and ribbon; plastic dyeing rack and dyeing vat.
Dyeing process
Section 65 °C, baking 1 h. The label: labeling according to the requirements, placing the slices in the dyeing dying rack. Placing according to the requirements of the Ventana Benchmark XT which requiring soak for 10% bovine 15 min (Nestle milk), water rinse. The primary antibody, DAB kit, hematoxylin, back to blue liquid on the reagent shelf, attention, bubble label row clean dry reagent sample in water. Add the rest of the required reagents, such as LCS, EZ Prep, Reaction Buffer, SSC*2, CC1, etc. Click on the “RUN” icon and complete the information confirmation before the operation; ALK automatic dyeing.
Immunohistochemical interpretation
First, slices were quality control evaluated through 2 slices to detect. Both 2 slices showed proper staining before they could interpret the current case. The tissue microarray stained with 1 g, a rabbit clone negative quality control, must be negative staining except for specific background staining. The result uses two-item pronunciation to judge, ALK positive: there is strong granular cytoplasmic staining in cancer cells (any percentage of positive tumor cells). Nonspecific staining factors should be excluded; ALK negative: there is no strong granular cytoplasmic staining in tumor cells.
Statistical analysis
Using statistical methods (enumeration data using chi square test) to analysis the results of the inter group rate and using spearman to analysis correlation.
Results
EGFR Mutation in NSCLC patients
Three hundred cases of NSCLC patients, including EGFR gene mutation 130 (130/300, 43.33%); exon 21 mutation (L858R) 58 (58/130, 44.62%), exon 21 mutation (L861Q) (1/130, 0.77%), exon 19 deletion mutation (69/130, 53.08%), 19del + 20T790M double mutant 1 (1/130, 0.77%), 20T790M + L858R double mutant 1 (1/130, 0.77%).
Relationship between EGFR gene mutation and clinical features
EGFR gene mutation 130, including female 67 (67/122, 54.92%), male 63 (63/178, 35.39%), the mutation rate of female patients is higher than male patients (54.92% vs. 35.39%, P<0.05); the mutation rate of smoking patients is lower than non-smoking patients (28.95% vs. 52.15%, P<0.05), the mutation rate of adenocarcinoma is higher than non-adenocarcinoma (49.37% vs. 19.67%, P<0.05), the mutation rate of advanced age (≥60 years) is higher than low age (<60 years) (46.53% vs. 40.1%, P>0.05), the mutation rate of Han patients is lower than Zhuang patients (41.60% vs. 46.43%, P>0.05); the mutation rate of right lung is lower than left lung (39.88% vs. 48.41%, P>0.05), the mutation rate of stages I and II is lower than stage III and IV (37.88% vs. 48.05%, P>0.05),the difference was not statistically significant.
Expression of EML4-ALK
Three hundred cases of NSCLC patients, EML4-ALK positive 13 cases (13/300, 4.33%), including male 9, female 4. The age range 33–74 years, the average age 51.46 years, ≥60 years: 3 cases, <60 years: 10 cases; no-smoking: 11 cases, smoking: 2 cases; adenocarcinoma 13 cases, alveolar type 2 cases, papillary type 1 case, the main tubular type 1 case, adherent type 2 cases, mucinous adenocarcinoma 2 cases, mixed type adenocarcinoma 5 cases; stage I 3 cases, stage II 3 cases, stage III 4 cases, stage IV 3 cases; lymph node metastasis 10 cases, no-lymph node metastasis 3 cases. ALK rearrangement of NSCLC was not significantly correlated with sex, age, smoking history, histological stage, histological type and lymph node metastasis (P>0.05), and there was no significant difference between the two groups.
Relationship of expression levels of EML4-ALK and EGFR in NSCLC patients
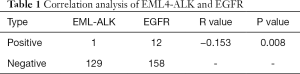
EGFR gene mutation 130, including ALK rearrangements 1 (1/130, 0.77%). EGFR wild-type 170, including ALK rearrangements 12 (12/170, 7.06%), EGFR gene mutations coexist with EML4-ALK positive expression 1, the application of spearman analysis, there was a negative correlation between the two group (R= −0.153, P=0.008), the difference was statistically significant. But fewer samples, the relationship between EGFR and ALK positive mutation to increase the sample size to further verify (see Table 1).
Full table
Discussion
In the past 10 years, molecular targeted therapy has achieved a surprising effect in the treatment of NSCLC, and greatly promoted the targeted treatment of NSCLC. In 2007, Soda (2) in NSCLC was first found in animal echinoderm microtubule associated protein 4-anaplastic lymphoma kinase (EML4-ALK) in tumor samples fusion gene. The positive rate of EML4-ALK fusion gene in NSCLC is 0.4–11.6%, average 3.4% (2-5), the expression rate of the eastern countries, Korea, Japan and China respectively was 3.6%, 2.9–11.6% and 2–6.7% (5-7), and the expression rate of the western countries, Caucasus was 0.4–2.7% (4). The total positive rate of Europe was 3.2% (65/2,011). There is no significant difference in the overall positive rate of EML4-ALK between eastern and Western populations. In this study, when the clinical pathological features of the subjects were not screened, the positive rate of ALK fusion gene was 4.33% (13/300), which was basically consistent with the overall positive rate in Asia. According to the report, EML4-ALK fusion genes are more prevalent in youth, nonsmoking or less smoking, women in NSCLC patients (8). The study found that female with the ALK mutation rate was 3.28% (4/122) lower than male (5.06%); no-smoking with the ALK mutation rate was 5.91% (11/186) higher than smoking (1.75%), the aged (≥60 years) with the ALK mutation rate was 2.08% (3/144) lower than the aged (<60 years) 6.41% (10/156), The difference was not statistically significant (P>0.05). The reports were small sample. The pathological types of 13 ALK positive patients were adenocarcinoma, consistent with the reports.
With clinical features of EGFR mutations, reports have shown that EGFR mutations rate was about 30% in China NSCLC patients. Female, no-smoking were more common (2,9-10). The data showed that the mutation rate of EGFR in NSCLC was 44.29% (128/289). Female 55.56% (65/117), adenocarcinoma 50.2% (116/231), and no-smoking 53.07% (95/179). The relationship between EGFR mutations and ALK positivity, many reports show that EGFR mutations are mutually exclusive and not coexisting with ALK positive (11). But there was also exception, China scholar Zhang (12) also reported the detection of double mutations of exons EGFR-19 and EML4-ALK in female patients with adenocarcinoma in pathological specimens. In this study, 1 cases of EGFR mutation and ALK positive coexistence were found. The mutation type of EGFR was 19-del deletion mutation. Application Spearman analysis showed that there was a negative correlation between the two (R= −0.153, P=0.008). At present, these common mutations are still rare events, but the relationship between EGFR and EML4-ALK in NSCLC needs to be further confirmed.
At present, the treatment of all cancer such as NSCLC, hepatocellular carcinoma (13,14), has entered individualized treatment. For the targeted treatment of NSCLC, should follow the order of EGFR and ALK, and according to the mutation, we should give the targeted drug treatment. ALK inhibitors, crizotinib, and two generation Ceritinib bring new hope for ALK positive NSCLC patients. With the development of new molecular targets for NSCLC and the continuous development of targeted drugs, individualized targeted therapies (15) will have a broader prospect.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.10.36). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study is approved by Wu Jieping Medical Foundation (No. 320.6750.14306). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [Crossref] [PubMed]
- Takeuchi K, Choi YL, Togashi Y, et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res 2009;15:3143-9. [Crossref] [PubMed]
- Martelli MP, Sozzi G, Hernandez L, et al. EML4-ALK rearrangement in non-small cell lung cancer and non-tumor lung tissues. Am J Pathol 2009;174:661-70. [Crossref] [PubMed]
- Wong DW, Leung EL, So KK, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 2009;115:1723-33. [Crossref] [PubMed]
- Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res 2008;14:4275-83. [Crossref] [PubMed]
- Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod Pathol 2009;22:508-15. [Crossref] [PubMed]
- Kim Y, Hida T, Nokihara H, et al. MA07.03 Alectinib (ALC) versus Crizotinib (CRZ) in ALK-Positive Non-Small Cell Lung Cancer (ALK+ NSCLC): Primary Results from Phase III Study (J-ALEX). J Thorac Oncol 2017;12.
- Oda N, Ichihara E, Hotta K, et al. Phase II Study of the EGFR-TKI Rechallenge With Afatinib in Patients With Advanced NSCLC Harboring Sensitive EGFR Mutation Without T790M: Okayama Lung Cancer Study Group Trial OLCSG 1403. Clin Lung Cancer 2017;18:241-4. [Crossref] [PubMed]
- Cortinovis D, Gregorc V, Migliorino MR, et al. New perspectives in the second-line treatment of non squamous NSCLC patients: Results from a large Italian Lung Cancer Working Group. Crit Rev Oncol Hematol 2017;109:35-41. [Crossref] [PubMed]
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189-97. [Crossref] [PubMed]
- Zhang X, Zhang S, Yang X, et al. Fusion of EML4 and ALK is associated with development of lung adenocarcinomas lacking EGFR and KRAS mutations and is correlated with ALK expression. Mol Cancer 2010;9:188. [Crossref] [PubMed]
- Medhat E, Esmat G, Hamza E, et al. Ophthalmological side effects of interferon therapy of chronic hepatitis C. Hepatobiliary Surg Nutr 2016;5:209-16. [Crossref] [PubMed]
- Obuch JC, Wagh MS. Endoscopic therapy for benign biliary strictures: evaluation of metal vs. plastic biliary stents. Hepatobiliary Surg Nutr 2017;6:268-71. [Crossref] [PubMed]
- Zhang L, Li G. Comment on “combination treatment including targeted therapy for advanced hepatocellular carcinoma”. Hepatobiliary Surg Nutr 2016;5:444-6. [Crossref] [PubMed]


