
Inhibition of ERK signaling potentiates the anti-tumor activity of FL118 on hepatocellular carcinoma cells
Introduction
Hepatocellular carcinoma (HCC), one of the most widespread cancers with the characteristic of high incidence and mortality, is the third leading factor of cancer-related death in the world (1). Except for sorafenib, effective molecule-targeting medication is still lacked for advanced HCC. As an inhibitor of extracellular regulated protein kinase (ERK), sorafenib greatly improves the survival of liver cancer patients, and even, is the only choice for late liver cancer therapeutics (2,3). However, it is limited to improve 5-year patient survival due to the inevitable drug resistance (4).
FL118, also known as 10, 11-methylenedioxy-20(S)-camptothecin, 10, 11-MD-CPT, MDCPT (5), and 10, 11-mCPT (6), is a novel camptothecin derivative initially identified as a potent inhibitor of survivin, a member of IAP family. Recent studies have reported that FL118 is able to inhibit the expression of anti-apoptotic factors, such as X-linked inhibitor of apoptosis protein (XIAP), Baculoviral IAP repeat-containing protein 2 (cIAP2) and Induced myeloid leukemia cell differentiation protein 1 (Mcl-1), and induce the expression of pro-apoptotic proteins, such as Bcl-2-associated X protein (Bax) and bisindolylmaleimide inhibitor (Bim) in various cancer cell types (7). Interestingly, FL118 exerts its excellent anticancer activity regardless of p53 status. In cancer cells with wild type p53, FL118 induces cancer cell apoptosis by quickly activating p53 pathway, while in cells with null or mutant p53, FL118 can target survivin, XIAP, clAP2 and Mcl-1 to suppress cell growth (7). FL118 is observed to lack of Top 1 (Topoisomerase)—inhibiting activity compared with other camptothecin analogues, such as topotecan and irinotecan. However, experimental studies showed that FL118 significantly reduces cell proliferation and tumor growth in many types of cancer in vitro and in vivo (7,8). These dates suggested that FL118 may employ a different mechanism other than from Top 1 inhibition to exert its antitumor activity. Furthermore, it is worth noting that FL118 exhibits low toxicity due to targeting of FL118 to antiapoptotic proteins, such as survivin, XIAP, clAP2 and Mcl-1, which are expressed at a lower level in normal tissue compare with in cancerous cells (7).
ERK, a member of mitogen-activated protein kinases (MAPK) signaling pathway, plays an essential role in the pathogenesis of human cancer, under both pathological and physiological conditions. It has been found that ERK signaling can influence many functions of cancer cells, such as cellular proliferation, apoptosis, differentiation and survival (9). ERK pathway is closely associated with apoptosis by affecting the activity of apoptosis-related proteins, such as Bax, Bim and caspase 9 (1,10). Increasing evidence has indicated that FL118 plays an antitumor role by targeting apoptosis-related pathways. However, the relation between ERK and antitumor activity of FL118 still remains unclear so far.
Based on the previous studies, we speculated that ERK pathway may be involved in the anti-tumor effect of FL118. In this work, we tested the activation of ERK pathway in the treatment of FL118 in human liver cancer cells and explored the tumor suppressing effect of combined therapy of ERK inhibitor and FL118.
Methods
Cell culture
PLC and Huh7 cells were from Cell Bank of Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China) and were cultured in DMEM with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. Cells were cultured in a humidified 37 °C condition with 5% CO2. All cells lines were cultured with the same conditions.
Compounds
FL118 were obtained from Dr. Li research group, ERK inhibitor U0126 were purchased from Selleckchem (Shanghai, China). FL118 was dissolved in DMSO to make stock solution (1 mM) which was diluted into needed concentration when used. U0126 (10 µM) that selectively blocked ERK was added to cells with FL118 (10 and 100 nM) together.
MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay
The viability of PLC and Huh7 cells that treated with administration concentration of FL118 was determined by employing MTT assay. PLC (4×103/well) and Huh7 (5×103/well) cells were seeded into 96-wells plates incubated in 5% CO2 at 37 °C for 12 h, and then treated with appropriate concentrations of FL118 or U0126 for 24, 48, and 72 h, respectively. Subsequently, the diluent MTT with 5 mg/mL was added into 96-well plates. The cells were culture for 4 h, then MTT was aspirated off and 200 µL DMSO was added to dissolved formazan crystals in each well. The plates were agitated for 30 s and the absorbance in each well was detected at 490 nm using Microplate Reader (Tecan, Shanghai, China).
Western blot analysis
The cells were washed twice with PBS, and then lysed on ice radio immunoprecipitation assay (RIPA) lysis buffer with protease inhibitors phenylmethanesulfonyl fluoride (PMSF) and all-in-one (Solarbio, Beijing, China). The concentration of total proteins was determined by BCA Protein Quantification Kit (Solarbio, Beijing, China). The proteins sample was separated by 15% SDS-PAGE gels, transferred to 0.45 micro polyvinyl difluoride (PVDF) membranes used semi-dry electrophoretic transfer, and then 5% skimmed milk in TBST was used to block the nonspecific binding site of PVDF membranes for 1h at room temperature. The membranes were incubated with antibodies which diluted with 5% skim milk at 4 °C overnight. Subsequently, the membranes were washed 3 times with TBST and then incubated with appropriate secondary antibodies for 2 h at room temperature. eECL western blot Kit (CWBIO, Beijing, China) was used to detect the protein binds. The antibodies involved in this study are as follows: ERK (Cell Signaling Technology, Boston, MA, USA), p-ERK (Cell Signaling Technology, Boston, MA, USA), GAPDH (Abcam, Cambridge, MA, USA).
Cell apoptosis assay
PLC and Huh7 cells were seeded into 6-well plates, the cells were divided into different groups randomly. Subsequently, after treated with appropriate concentration of FL118 or U0126 for 24 h, cells were washed with PBS and then incubated with Hoechst and propidium iodide (PI). Four random four file of view demonstrating total cells (Hochest-stained nuclei) or death (PI-stained nuclei) cells were observed by microscope (Nikon, Beijing, China). In each plate the percentage (PI/Hoechst) of cell apoptosis was determined by averaging of four files.
Statistical analysis
All the experiments were conducted for at least three times except western blot assays. All date are shown as means ± SD in figures. The samples’ difference was analyzed by one-way ANOVA, statistical significance was considered as: *, P<0.05; **, P<0.01; ***, P<0.001; ****, P<0.0001; and ns, no significant difference.
Results
FL118 inhibits the viability of PLC and Huh7 cells
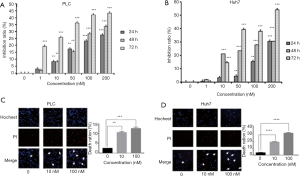
Different concentrations (0, 1, 10, 50, 100 and 200 nM) of FL118 was administrated on PLC and Huh7 cells for 24, 48, and 72 h. Cell viability was detected by MTT assay. As shown in Figure 1A,B, FL118 dramatically decreased the viability of PLC and Huh7 cells in a dose-dependent manner.
FL118 induces apoptosis of PLC and Huh7 cells
To observe the effect of FL118 on apoptosis of human liver cancer cells, PLC and Huh7 cells were treated with different concentrations (10 and 100 nM) of FL118 for 24 h. Apoptosis was assessed by Hochest-PI staining. As shown in Figure 1C,D, exposure to both 10 and 100 nM FL118 led to a significant apoptosis in cancer cells. Few apoptotic cells were observed in control groups. However, some apoptotic cells were detected under FL118 treatment and apoptotic events were found to be increased along with the elevated concentrations.
FL118 induces ERK activation in cancer cells in a time-dependent manner
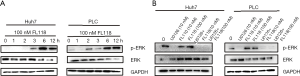
Subsequently, to explore the potential anticancer activity of FL118 on liver cancer cells, we investigated if ERK pathway was activated after treatment with 100 nM FL118. Western blotting was used to detect the expression of total and phosphorylated ERK proteins at 0, 1, 2, 3, 6, 12 h. The results showed treatment of 100 nM FL118 decrease and increase the level of total and phosphorylated ERK proteins in a time-dependent manner, respectively (Figure 2A), indicating that FL118 can inhibit the ERK pathway, and that PLC and Huh7 cells may resist against FL118’s anticancer potency via activating ERK pathway. Subsequently, we detected the expression of total and phosphorylated ERK after treatment with FL118 or/and U1026, an ERK-specific inhibitor, for 6 h. As indicated in Figure 2B, there is no significant changes in the level of total ERK protein between FL118 alone and combined treatment of FL118 and 10 µM U0126. However, the combined administration of U0126 and FL118 led to a significant suppression of ERK phosphorylation, compared with FL118 alone. These results indicated that FL118 treatment induce the activation of ERK pathways in liver cancer cells.
The capability of FL118 to inhibit cell viability and induce apoptosis is potentiated by suppression of ERK activation in cancer cells
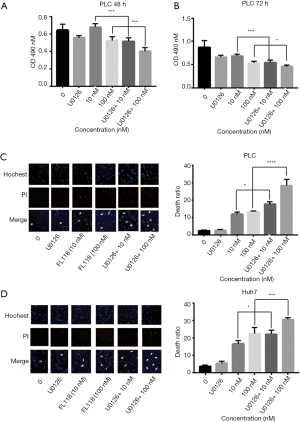
To further determine the relationship between FL118 and ERK pathway, we detected the cell viability and apoptosis in PLC cells after the treatment with U0126 and FL118. Figure 3A,B showed that combined treatment of U0126 and FL118 significantly inhibited the growth of both PLC cells, compared to control and FL118 groups. Moreover, Figure 3C,D demonstrated that the apoptotic ratio was obviously higher in PLC and Huh7 cells after combined treatment with FL118 and U0126 for 24 h than that with only FL118 stimulation. The above results indicated that the combined therapy using both ERK inhibitor and FL118 might be more effective than FL118 alone for inhibiting proliferation and inducing apoptosis in PLC and Huh7 cells.
Discussion
HCC is one of most common malignant tumors with the characteristics of high incidence, frequent relapse and poor prognosis (11). According to previous studies, liver cancer is the fifth and the seventh most common cancer in men and women, respectively (1). Currently, chemotherapy plays an important role in the treatment of patients who are not willing to or suitable for surgery (12). However, the occurrence of drug resistance limits the effect of chemotherapy (4).
Hinted by the resistance of cancer to drugs, it appears not to be an effective way to target a single molecular pathway for eliminating tumor cells. Therefore, it is believed to be a more effective strategy to target multiple cancer-associated signaling pathways. A newly-developed camptothecin analogue, FL118, has been observed to inhibit the growth of colon and head-neck cancer with a lower toxicity in mice models, compared with irinotecan or its active metabolite, SN-38 (7,13,14). ABCG2 (15), a drug efflux pump (16), plays a pivotal role in cancer resistance to drugs, such as irinotecan, SN-38 (17), methotrexate (18) and a variety of tyrosine kinase inhibitors (19,20). Intriguingly as a poor substrate for ABCG2, FL118 displays better therapeutic efficacy in colon and lung cancer models than irinotecan through bypassing the ABCG2-induced drug resistance. Different from other camptothecin analogues, the antitumor ability of FL118 is irrelevant to the suppression of Top 1 enzyme activity (21). Thus, these data demonstrated that FL118 has a unique mechanism against cancer. In a previous study, FL118 exhibited strong anticancer activity, low toxicity and drug resistance in colon and head-neck cancer (8). We aimed to detect if FL118 has anticancer activity on human liver cancer cells and to create a potential rationale for its clinical application. Our results showed that FL118 reduced cell viability and induced apoptosis in dose- and time-dependent manners in PLC and Huh7 cells.
Accumulated evidence has demonstrated that dysregulated apoptosis is closely associated with initiation and progression of cancers (12). MAPK signaling cascade has been indicated to mediate cellular response to the intracellular and extracellular stimuli (22). Among the members of MAPKs, ERK is usually associated with cell apoptosis (23). Many studies have elucidated that ERK signaling is able to induce apoptosis in cancer cells (24). Experimental studies demonstrated that ERK pathway activation influences the expression of survivin, and thus to inhibit apoptosis in human cancer cells (25). Furthermore, other recently studies showed that the ERK pathway in conjugation with AKT regulated survivin activation on cancer cell lines (26). Based on the relationship between FL118 and survivin, ERK signaling might be an important pathway to FL118’s anticancer effect. However, it is still yet to be determined if ERK pathway is affected by the treatment of FL118. Thus, we further detected the status of ERK pathway after treatment of FL118. Our results showed that the phosphorylation of ERK is enhanced after treatment of FL118 in PLC and Huh7 cells. Notably, the level of total ERK remained unchanged. Taken together, we found that FL118 induced the activation of ERK signaling pathway in liver cancer cells. In our work, it is the first time to demonstrate that FL118 is associated with ERK pathway in tumor cells. As reported previously, camptothecin can activate the ERK pathway through influence its upstream proteins (27). Some studies showed that camptothecin activates ERK by suppressing mitogen-activated protein kinase phosphatase-1 (MKP1) in various types of cancer cells (27). Based on the structural similarity between camptothecin derivatives, FL118 may also exert its effect on the activation of ERK pathway through MKP1, However, the mechanism by which FL118 activates the ERK pathway is still unfounded so far. And more, it should be noted that this finding is inconsistent with our initial speculation, because ERK activation is well believed to decrease apoptosis in cancer cells. These data suggested that FL118 may utilize a molecular signaling other than ERK pathway to exert its ability to trigger apoptosis. We will search for the exact molecular mechanism by which FL118 leads to the apoptosis in these malignant cells in further work.
Subsequently, it is interesting to us if the proapoptotic activity of FL118 can be further potentiated by the suppression of ERK pathway. Our results showed that suppression of ERK pathway can enhance the apoptosis and inhibit cell viability after treatment of FL118 in PLC and Huh7 cells, indicating that abrogation of ERK signaling is indeed an effective strategy to further enhance the anticancer activity of FL118. Previous studies have also reported that ERK signaling is activated during the administration of anticancer compounds for clinical therapeutics (28), which is consistent with our results. And this phenomenon is believed to probably induce the drug resistance of cancer cells to this antitumor molecule.
Intriguingly, some studies demonstrated that the activation of ERK signaling can be inhibited by some natural products, such as celastrol (29) and curcumin (30), which may also be used to enhance the anticancer effect of FL118. Although ERK activation is involved in the impairment of FL118’s antitumor efficacy, it’s still worthy of being further investigated if other possible mechanisms are associated with a weakened antitumor activity of FL118. It is an effective strategy to improve the clinical outcomes of FL118 by targeting these signaling pathways.
Acknowledgments
We would like to thank Fengzhi Li from Roswell Pk Canc Inst and Dept Pharmacol & Therapeut for supporting.
Funding: This work was supported by funds from National Natural Sciences Foundation of China (81502065 and 81672926), China Postdoctoral Science Foundation Funded Project (2016T90613, 2015M580574 and 2016M592146), Natural Sciences Foundation of Shandong Province (ZR2014HQ009), Shandong Postdoctoral Innovation Project (201602037), Qingdao Innovation Applied Basic Research Project (16-5-1-56-JCH) and Qingdao Postdoctoral Research Project (2015167 and 2015157).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.10.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264-73.e1. [Crossref] [PubMed]
- Liu L, Cao Y, Chen C, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res 2006;66:11851-8. [Crossref] [PubMed]
- Lo J, Lau EY, Ching RH, et al. Nuclear factor kappa B-mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice. Hepatology 2015;62:534-45. [Crossref] [PubMed]
- De Giorgio M, Fagiuoli S. Management of hepatocellular carcinoma. Dig Dis 2007;25:279-81. [Crossref] [PubMed]
- Wadkins RM, Bearss D, Manikumar G, et al. Hydrophilic camptothecin analogs that form extremely stable cleavable complexes with DNA and topoisomerase I. Cancer Res 2004;64:6679-83. [Crossref] [PubMed]
- Knab AM, Fertala J, Bjornsti MA. A camptothecin-resistant DNA topoisomerase I mutant exhibits altered sensitivities to other DNA topoisomerase poisons. J Biol Chem 1995;270:6141-8. [Crossref] [PubMed]
- Ling X, Cao S, Cheng Q, et al. A novel small molecule FL118 that selectively inhibits survivin, Mcl-1, XIAP and cIAP2 in a p53-independent manner, shows superior antitumor activity. PLoS One 2012;7:e45571 [Crossref] [PubMed]
- Li F. Anticancer drug FL118 is more than a survivin inhibitor: where is the Achilles' heel of cancer? Am J Cancer Res 2014;4:304-11. [PubMed]
- Vitagliano O, Addeo R, D'Angelo V, et al. The Bcl-2/Bax and Ras/Raf/MEK/ERK signaling pathways: implications in pediatric leukemia pathogenesis and new prospects for therapeutic approaches. Expert Rev Hematol 2013;6:587-97. [Crossref] [PubMed]
- McCubrey JA, Steelman LS, Chappell WH, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 2007;1773:1263-84. [Crossref] [PubMed]
- Yu G, Jing Y, Kou X, et al. Hepatic stellate cells secreted hepatocyte growth factor contributes to the chemoresistance of hepatocellular carcinoma. PLoS One 2013;8:e73312 [Crossref] [PubMed]
- Yu R, Yu BX, Chen JF, et al. Anti-tumor effects of Atractylenolide I on bladder cancer cells. J Exp Clin Cancer Res 2016;35:40. [Crossref] [PubMed]
- Ling X, Li F. An intravenous (i.v.) route-compatible formulation of FL118, a survivin, Mcl-1, XIAP, and cIAP2 selective inhibitor, improves FL118 antitumor efficacy and therapeutic index (TI). Am J Transl Res 2013;5:139-54. [PubMed]
- Zhao J, Ling X, Cao S, et al. Antitumor activity of FL118, a survivin, Mcl-1, XIAP, and cIAP2 selective inhibitor, is highly dependent on its primary structure and steric configuration. Mol Pharm 2014;11:457-67. [Crossref] [PubMed]
- Westover D, Ling X, Lam H, et al. FL118, a novel camptothecin derivative, is insensitive to ABCG2 expression and shows improved efficacy in comparison with irinotecan in colon and lung cancer models with ABCG2-induced resistance. Mol Cancer 2015;14:92. [Crossref] [PubMed]
- Doyle LA, Yang W, Abruzzo LV, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A 1998;95:15665-70. [Crossref] [PubMed]
- Yang CH, Schneider E, Kuo ML, et al. BCRP/MXR/ABCP expression in topotecan-resistant human breast carcinoma cells. Biochem Pharmacol 2000;60:831-7. [Crossref] [PubMed]
- Volk EL, Schneider E. Wild-type breast cancer resistance protein (BCRP/ABCG2) is a methotrexate polyglutamate transporter. Cancer Res 2003;63:5538-43. [PubMed]
- Honjo Y, Hrycyna CA, Yan QW, et al. Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells. Cancer Res 2001;61:6635-9. [PubMed]
- Ozvegy-Laczka C, Hegedus T, Varady G, et al. High-affinity interaction of tyrosine kinase inhibitors with the ABCG2 multidrug transporter. Mol Pharmacol 2004;65:1485-95. [Crossref] [PubMed]
- Hertzberg RP, Caranfa MJ, Hecht SM. On the mechanism of topoisomerase I inhibition by camptothecin: evidence for binding to an enzyme-DNA complex. Biochemistry 1989;28:4629-38. [Crossref] [PubMed]
- Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta 2010;1802:396-405. [Crossref] [PubMed]
- Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer 2009;9:537-49. [Crossref] [PubMed]
- Liu J, Ma L, Chen X, et al. ERK inhibition sensitizes cancer cells to oleanolic acid-induced apoptosis through ERK/Nrf2/ROS pathway. Tumour Biol 2016;37:8181-7. [Crossref] [PubMed]
- Kanwar JR, Kamalapuram SK, Kanwar RK. Targeting survivin in cancer: the cell-signalling perspective. Drug Discov Today 2011;16:485-94. [Crossref] [PubMed]
- Ye Q, Cai W, Zheng Y, et al. ERK and AKT signaling cooperate to translationally regulate survivin expression for metastatic progression of colorectal cancer. Oncogene 2014;33:1828-39. [Crossref] [PubMed]
- Lee M, Young Kim S, Kim J, et al. Mitogen-activated protein kinase phosphatase-1 inhibition and sustained extracellular signal-regulated kinase 1/2 activation in camptothecin-induced human colon cancer cell death. Cancer Biol Ther 2013;14:1007-15. [Crossref] [PubMed]
- Ko JK, Auyeung KK. Target-oriented mechanisms of novel herbal therapeutics in the chemotherapy of gastrointestinal cancer and inflammation. Curr Pharm Des 2013;19:48-66. [PubMed]
- Ma J, Han LZ, Liang H, et al. Celastrol inhibits the HIF-1alpha pathway by inhibition of mTOR/p70S6K/eIF4E and ERK1/2 phosphorylation in human hepatoma cells. Oncol Rep 2014;32:235-42. [Crossref] [PubMed]
- Lim JH, Kwon TK. Curcumin inhibits phorbol myristate acetate (PMA)-induced MCP-1 expression by inhibiting ERK and NF-kappaB transcriptional activity. Food Chem Toxicol 2010;48:47-52. [Crossref] [PubMed]


