
miRNA-451 inhibits proliferation and motility of the gastric cancer SGC-7901 cell line via targeting AKT-mediated signal pathway
Introduction
GC is the second most common cause of cancer-related death worldwide. It has been estimated that approximately 1 million patients are newly diagnosed with gastric cancer worldwide each year, which accounts for nearly 10% of all cancer deaths (1,2). Patients with gastric cancer are diagnosed mostly at advanced clinical stages, typically accruing lymphatic tumor dissemination, with poor prognosis and a 5-year survival rate <30% (3). The tumorigenesis and development of GC is multifactorial and complicated which evolves in dysregulation of various genetic and epigenetic alterations (4). However, the detailed molecular mechanisms in GC have not been well elucidated at present. Therefore, it is of great significance to explore the molecular mechanisms underlying the initiation and progression of GC in order to develop novel and efficient therapeutic strategies for GC.
miRNAs are a class of small, endogenous non-coding RNAs that have been identified as post- transcriptional negative regulators of gene expression (5). miRNAs are predicted to regulate the expression of approximately one-third of all human genes (6,7). Accumulating evidences suggest that abnormally expressed miRNAs has been identified in various types of human malignant tumors, and their expression was significantly correlated with the initiation and progression of these cancer types (8).
In this study, we examined miRNA-451 expression at tissue and cell level. Meanwhile, we further evaluated the effects of miRNA-451 on the biological behavior of SGC-7901 cells and then explored the molecular mechanisms underlying miRNA-451-inhibited growth and metastasis of SGC-7901 cells.
Methods
Tissues specimens
A total of 38 pairs of matched gastric carcinoma tissues and the matched tumor-adjacent tissues (resected samples 3–5 cm from the carcinoma tissues) were procured from the surgical resection specimens of department of general surgery, Renmin Hospital of Wuhan University from 2012 to 2013. All patients received no treatment before surgery. The collected specimens were immediately stored at −80 °C until use. All patients signed informed consent forms for sample collection. The use of patient samples, which comprised the tumor and the adjacent normal tissues, was approved by our institutional ethics committee.
Cell culture
Human gastric cell lines SGC-7901 and GES-1 epithelial cells were obtained from the center for Type Culture Collection and routinely maintained in Dulbecco’s modified Eagle’s medium (In-vitrogen Life Technologies, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S) at 37 °C in humidified air containing 5% carbon dioxide air atmosphere.
Cell transfection
miRNA-451 mimics and negative control miRNA mimics(miRNA-NC) bought from Ruibo company (Guangzhou, China) were transfected into the SGC-7901 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. After 6 h, the medium was changed to complete medium, and cells were cultured at 37 °C in 5% CO2. The negative control mimics was labeled with the Silencer 6-carboxy-fluo-rescine (FAM), Transfection efficiency was estimated according to the density of cell glowing red florescence at 24 h after transfection.
Real time PCR analysis
Total RNA from tissue and cells was extracted using Trizol (Invitrogen) according to the manufacturer’s instructions. RNA concentration and purity was detected with spectra-photometer. Reverse-transcribed complementary DNA was synthesized with the Prime-Script RT reagent Kit (TaKa-Ra Dalian, China). Real time polymerase chain reaction (PCR) was performed with SYBR Pre-mix ExTaq (TaKaRa, Dalian, China). The RT and PCR primers for miRNA-451 and U6 were purchased from Ruibo company (Guangzhou, China).
miRNA-451:
Sense, 5’-ACACTCTGGGAAA TACCATTACT-3’ Revere, 5’-CTGGTGTCGTGGAGTCGGCAA-3’ U6: Sense, 5’-CTCGCTTCGGCAGCACA-3’ Reverse, 5’-AACGCTTCACGAATTTGCGT-3
For the reverse transcription (RT) reactions, RT reactions were performed at 42 °C for 60 min, 70 °C for 10 min and then maintained at 4 °C. After a sufficient amount of cDNA was obtained, PCR amplification was performed using a real-time PCR cycler (7500ABI, USA). The amplification reaction system (20 µL) included SYBY Green Mix (9 µL), RT product (2 µL), Bulge-LoopTM miRNA Forward Primer (2 µL), Bulge-LoopTM miRNA Reverse Primer (2 µL) and ddH2O (5 µL). The reaction conditions were as follows: Stage 1, 95 °C for 20 s (1 cycle); Stage 2, 95 °C for 10 s followed by 60 °C for 20 s, 70 °C for 5 s (40 cycles); the PCR primers for P21, P27, C-myc, CyclinD1, Bcl-2, Bax, MMP-2, MMP-9 and AKT were synthesized by Shanghai Sangon Company. The annealing temperatures for PCR reactions of those genes are 55–56 °C. The results of real-time PCR were analyzed by the DDCt method: ΔCT = CTselected gene − CTreference gene, ΔΔCT = ΔCTexperimental group − ΔCTcontrol group, RQ experimental group = 2 − ΔΔCT, RQcontrol group =1. The results of real-time PCR were presented as the ratio between the selected genes and reference transcripts.
Cell counting Kit-8 assay
The effects of miRNA-451 on cell proliferation were assessed with the Cell Counting Kit-8 (CCK-8, Dojindo, Japan). Briefly, the cells were seeded into 96-well plates (0.8×104 cells/well) and allowed to attach overnight. After transfection, CCK-8 was added to each well at various time points (0, 24, 48, 72 and 96 h) and incubated at 37 °C for 0.5 h. The absorbance at 450 nm was measured using microplate reader. Three independent experiments were performed in quadruplicate.
Colony formation assay
Approximately 1×103 SGC-7901 cells were placed in a fresh 6-well plate overnight. Then, the cell was transfected with miRNA mimics for 6 h and then was maintaining in DMEM containing 10% FBS for 2 weeks. Colonies were fixed with 20% methanol for 15 min and stained with 0.1% crystal violet. The visible colonies were manually counted.
Cell-cycle analysis
Flow cytometry was used for cell cycle analysis. Cells were washed with phosphate-buffered saline (PBS), trypsinized and resuspended in medium. The supernatant was removed after centrifugation and the cells were fixed and permeabilized by 75% ethanol overnight at −20 °C and then incubated with 100 g/mL RNAase at 37 °C for 30 min. Nuclei of cells were then stained with 50 g/mL propidium iodide (PI) for 30 min. A total of 105 cell nuclei PI fluorescence were examined in a Flow Cytometer. The width and area of the PI fluorescence per cell were recorded for at least 104 cells per sample and DNA histograms were analyzed by Modifit software (Becton Dickinson). The percentage of cells in each phase of the cell cycle was analyzed by ModFit software.
Assessment of apoptosis
The annexin V-FITC Apoptosis Detection Kit I (Abcam, USA) was used to detect apoptosis. For flow cytometry assay, Cells were harvested by centrifugation for 5 min at 800 rpm/min. Cells were resuspended in 1× binding buffer, stained with FITC-labeled annexin V for 5 min and immediately analyzed by Flow Cytometer.
Cellular migration and invasion assays
Migration and invasion assays were carried out in a 24-well transwell plate with 8.0 µm pore inserts (Corning Costar, Lowell, MA, USA). Collagen type 1-coated inserts (0.5 mg/mL, BD Bioscience, San Jose, CA, USA) were used in the migration assay and matrigel-coated (1/15 dilution, BD Bioscience) inserts were used in the invasion assay. Two hundred µL conditioned medium with 10% FBS was used as chemoattractant and placed in bottom chamber. One hundred µL of serum free DMEM containing 1×105 cells were added to the upper chamber of the inserts and allowed to transmigrate or invade into the lower chamber. After incubation for 24 h at 37 °C in a CO2 incubator, Cells on the top surface of the insert were removed by wiping with a cotton swab, while cells migrating/invading to the bottom surface of the insert was washed with PBS, fixed with methanol, stained with 0.4% crystal violet solution and then subjected to a microscopic inspection at a 200× magnification. Then cells were counted within five randomly chosen fields.
Western blot analysis
Fifty micrograms of protein extracted were separated in a 10% SDS-polyacrylamide gel and transferred onto a PDVF membrane (Millipore, Nether-lands). Membranes were blocked with 5% non-fat dried milk for 1 h at room temperature and incubated with primary antibodies at 4 °C overnight. After washing with TBST, the blots were incubated for 1 h with horseradish peroxidase-conjugated secondary antibodies and visualized using the super ECL detection reagent (Applygen, Beijing, China).
Results
miRNA-451 is downregulated in human gastric cancerous tissues and SGC-7901 cells
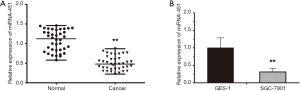
miRNA-451 expression levels was detected by qRT-PCR. Results showed that the expression of miRNA-451 were significantly downregrulated in gastric cancer tissues compared with matched para-cancerous tissue (Figure 1A). Similarly, the expression of miRNA-451 in SGC-7901 cells was dramatically lower compared with the expression levels in the normal gastric epithelium GES-1 cell line (Figure 1B). Collectively, these findings suggested that miRNA-451 was down- regulated in GC and might be associated with the carcinogenesis of GC.
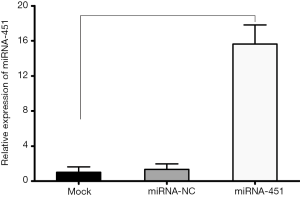
The expression of miRNA-451 in SGC-7901 cells after transfection
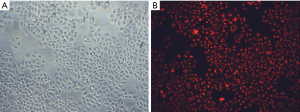
Transfection efficiency (>95%) was confirmed with the use of the Silencer FAM-labeled miRNA-NC mimics which glowing red fluorescent in cells at 24 h after transfection (Figure 2). Its expression was also measured using qRT-PCR assay. As shown in Figure 3, following transfection miRNA-451 was significantly upregulated in SGC-7901 cell lines. The above results indicated that miRNA mimics have been successfully transfected and expressed high-efficiently in SGC-7901 cells.
Upregulation of miRNA-451 inhibits gastric SGC-7901 cell proliferation
Cell survival was analyzed by CCK8 assay cell counting Kit-8 and colony formation assay. As presented in Figure 4A, overexpression of miRNA-451 inhibited the proliferation of SGC-7901 cells following transfection. Moreover, as shown in Figure 4B, the number of colonies formed from SGC-7901/miRNA-451 cells was significantly lower than that formed from mock SGC-7901 or SGC-7901/miRNA-NC cells. Thus, these findings suggested miRNA-451 could induce growth inhibition in SGC-7901 cell.

Upregulation of miRNA-451 induces SGC-7901 cell cycle arrest at the G0/G1 phase
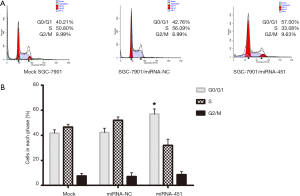
We performed flow cytometry analysis to explore whether miRNA-451 affected the cell cycle of SGC-7901 cells. As shown in Figure 5, the cell cycle in SGC-7901 cell was arrested in the G0/G1 phase following transfection with miRNA-451mimics in comparison with NC and mock groups, thus delaying the progression of cell cycle.
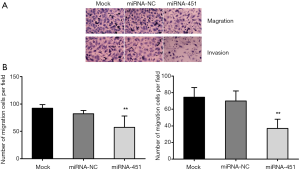
miRNA-451 suppressed GC cell migration and invasion
We used transwell assay to investigate whether miRNA-451 influenced migration and invasion on SGC-7901 cells. Cell migration and invasion potential was determined on the number of cells moving through microscope. As shown in Figure 6, the number of SGC-7901/miRNA-451 cells invading through the matrigel was significantly decreased from those of the mock or the miRNA-NC treated cells. Similar results were observed in migration assay, where the SGC-7901/miRNA-451 cells showed a significant reduction in ability to migrate through membranes that were not coated with Matrigel.
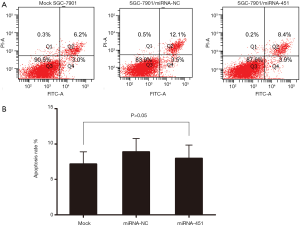
Upregulation of miRNA-451 didn’t induce the apoptosis of SGC-7901 cell
To measure the effect of miRNA-451 on apoptosis of SGC-7901 cell, we further conducted Flow Cytometric analysis assay. As shown in Figure 7, no significant difference in apoptosis rate was detected among those three groups (P>0.05).
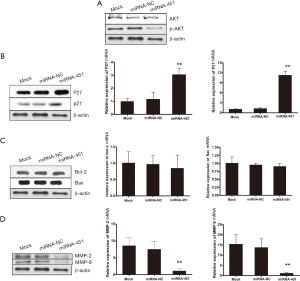
miRNA-451 inactivates the Akt signaling pathway and alters both cell cycle and metastasis-associated gene in post transcription level
It has been reported that activation of the Akt signaling pathway can regulate many biological phenomena of gastric cancer cells such as cell proliferation and survival, motility and migration. To explore the mechanisms by which miRNA-451 exerts its biological effects, we analyzed the effects of miRNA-451 on the Akt signaling pathway in SGC-7901 cells. Results showed that upregulation of miRNA-451 significantly downregulate the expression of pAkt protein but had no effects on the expression of total Akt protein (Figure 8A). Additionally, the expression of cell cycle and metastasis-associated gene varied with the overexpression of miRNA-451 at post-transcriptional levels. As shown in Figure 8B, the expression levels of P27/P21 mRNA and protein were significantly upregulated. However, the levels of endogenous Bax and Bcl-2 were unaffected by the upregulation of miRNA-451 (Figure 8C). Meanwhile, we showed that increasing miRNA-451 had significant effect on the expression of MMP-2 and MMP-9 at both mRNA and protein levels (Figure 8D). These results showed that AKT-mediated signal pathway might be potentially involved in miRNA-451-regulated SGC-7901 cells suppressing function.
Discussion
Gastric cancer is a highly aggressive and lethal malignancy. Gastric cancer carcinogenesis is a multi-step and multistage process, a number of molecules and complex regulatory networks are involved in gastric carcinogenesis, including activation of oncogenes, inactivation of cancer suppressor genes and changes in epigenetic modification (9).
miRNAs are a class of noncoding RNAs that function as negative regulators of gene expression . It was reported that the dysregulation of miRNAs might contribute to cancer progression by regulating multiple types of target genes expression (10-12). A number of miRNAs have shown to be involved in the progression of gastric cancer, For example, miRNA-203 is aberrantly down- regulated in gastric cancer, and suppresses the proliferation and invasiveness of gastric cancer cells by targeting Slug (13). While, miRNA-25 inhibits cell apoptosis of human gastric adenocarcinoma cell line AGS via regulating CCNE1 and MYC (14). As we known, the AKT signal pathway plays a critical role in controlling a range of diverse cellular functions (15,16) and the dysregulated PI3K/AKT pathway induced by some miRNA is involved in the onset and progression of cancer (17). Nan and his colleagues revealed that miRNA-451 impacts glioblastoma cell proliferation, invasion and apoptosis, perhaps via regulation of the PI3K/AKT signaling pathway (18). Wang et al. (19) also found that miRNA-451 could induce caspase-3-dependent apoptosis in NSCLC cells, which might be associated with inactivation of the Akt survival pathway. Bian et al. (20) also found that upregulation of miRNA-451 could significantly inhibit growth and enhance caspase-3 dependent apoptosis of A549 cells by inactivating the Akt signaling pathway. These results show that miRNA-451 may affect cancer-related biological processes via AKT signaling pathway.
In the present study, we first performed qPCR to detect the expression levels of miRNA-451 in 38 paired gastric carcinoma tissues and matched tumor adjacent tissues. It was demonstrated that miRNA-451 was significantly downregulated in GS tissues. Same results were observed in cell lines. The result implied that miRNA-451 might acted as an potential functional gene in the pathogenesis of gastric cancer. Thereafter, using a series of in vitro assays, we uncovered the biological functions of miRNA-451. The results of colony formation assay and CCK-8 assays showed overexpression of miRNA-451 in SGC-7901 cells resulted in suppression of cellular proliferation, the flow cytometry analysis further confirmed this effect. Moreover, transwell migration and Matrigel invasion assays were performed to explore whether miRNA-451 affected the migration and invasion capacity of SGC-7901 cells. As expected, transwell results showed that upregulation of miRNA-451 suppressed cell migration and invasion remarkably. However the apoptosis of SGC-7901 cells was not influenced. These results indicated that miRNA-451 might act as tumour suppressors in GC. In order to elucidate whether miRNA-451 could regulate the AKT signaling pathway and then perform above mentioned biological functions in gastric cancer, we detected the certain markers including total AKT, p-AKT, P21, CyclinD1, P27, C-myc, MMP2/9, Bcl-2 and Bax, all of which belong to the components of or correlated with the AKT signaling pathway. Intriguingly, western blot analysis and qPCR assays confirmed that upregulating miRNA-451 could inhibited AKT phosphorylation and subsequently reduced C-myc, MMP2, MMP9 mRNA and protein expression and increased P21, P27 mRNA and protein expression, although the total AKT, CyclinD1, Bcl-2 and Bax were not been changed. These results may explain why miRNA-451 could inhibit proliferation and motility of SGC-7901 cells.
In conclusion, the present study identified that miRNA-451 was downregulated in GC tissues and cells. Overexpression of miRNA-451 effectively inhibited the proliferation, migration and invasion of SGC-7901 cells. Furthermore, the AKT signaling pathway was demonstrated as a functional effect target of miRNA-451 in GC. In other words, miRNA-451 act as a tumor suppressor in the GC, and its effects are at least, in part, mediated through regulating Akt pathway. Objectively speaking, it is only a limited understanding of the complex relationship between the tumor cells and miRNA-451 and far from reaching a total understanding of the whole molecular picture of miRNA-451. The exact mechanisms of miRNA-451 need to be elucidated further in the future.
Acknowledgments
We are grateful to professor Dong Chen Wu for manuscript modification suggestions.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.11.18). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of Renmin Hospital of Wuhan University. All patients signed informed consent forms for sample collection.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol 2009;472:467-77. [Crossref] [PubMed]
- Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer 2000;88:921-32.
- Ali Z, Deng Y, Ma C. Progress of research in gastric cancer. J Nanosci Nanotechnol 2012;12:8241-8. [Crossref] [PubMed]
- Ambros V. The functions of animal miRNAs. Nature 2004;431:350-5. [Crossref] [PubMed]
- Mendell JT. miRiad roles for the miRNA-17-92 cluster in development and disease. Cell 2008;133:217-22. [Crossref] [PubMed]
- Chaudhuri K, Chatterjee R. MicroRNA detection and target prediction: integration of computational and experimental approaches. DNA Cell Biol 2007;26:321-37. [Crossref] [PubMed]
- Penna E, Orso F, Taverna D. miR-214 as a key hub that controls cancer networks: small player, multiple functions. J Invest Dermatol 2015;135:960-9. [Crossref] [PubMed]
- Tamura G, Yin J, Wang S, et al. E-Cadherin gene promoter hypermethylation in primary human gastric carcinomas. J Natl Cancer Inst 2000;92:569-73. [Crossref] [PubMed]
- Hsu KW, Fang WL, Huang KH, et al. Notch1 pathway-mediated miRNA-151-5p promotes gastric cancer progression. Oncotarget 2016;7:38036-51. [Crossref] [PubMed]
- Liu J, Xue H, Zhang J, et al. MicroRNA-144 inhibits the metastasis of gastric cancer by targeting MET expression. J Exp Clin Cancer Res 2015;34:35. [Crossref] [PubMed]
- Tan YY, Xu XY, Wang JF, et al. MiR-654-5p attenuates breast cancer progression by targeting EPSTI1. Am J Cancer Res 2016;6:522-32. [PubMed]
- Yang L, Liang H, Wang Y, et al. MiRNA-203 suppresses tumor cell proliferation, migration and invasion by targeting Slug in gastric cancer. Protein Cell 2016;7:383-7. [Crossref] [PubMed]
- Zhang Y, Peng Z, Zhao Y, et al. microRNA-25 Inhibits Cell Apoptosis of Human Gastric Adenocarcinoma Cell Line AGS via Regulating CCNE1 and MYC. Med Sci Monit 2016;22:1415-20. [Crossref] [PubMed]
- Brazil DP, Park J, Hemmings BA. PKB binding proteins. Getting in on the Akt. Cell 2002;111:293-303. [Crossref] [PubMed]
- Dufour G, Demers MJ, Gagné D, et al. Human intestinal epithelial cell survival and anoikis. Differentiation state-distinct regulation and roles of protein kinase B/Akt isoforms. J Biol Chem 2004;279:44113-22. [Crossref] [PubMed]
- Baik SH, Lee J, Lee YS, et al. ANT2 shRNA downregulates miR-19a and miR-96 through the PI3K/Akt pathway and suppresses tumor growth in hepatocellular carcinoma cells. Exp Mol Med 2016;48:e222 [Crossref] [PubMed]
- Nan Y, Han L, Zhang A, et al. MiRNA-451 plays a role as tumor suppressor in human glioma cells. Brain Res 2010;1359:14-21. [Crossref] [PubMed]
- Wang R, Wang ZX, Yang JS, et al. MicroRNA-451 functions as a tumor suppressor in human non-small cell lung cancer by targeting ras-related protein 14 (RAB14). Oncogene 2011;30:2644-58. [Crossref] [PubMed]
- Bian HB, Pan X, Yang JS, et al. Upregulation of microRNA-451 increases cisplatin sensitivity of non-small cell lung cancer cell line (A549). J Exp Clin Cancer Res 2011;30:20. [Crossref] [PubMed]


