
Integrated formulas to forecast prostate cancer: the parameters of influencing the prostate specific antigen level as an adjunct to prostate specific antigen and multi-parametric MRI to predict prostate cancer before biopsy
Introduction
In the USA, the highest incidence rate of malignant tumor is Pca in men, and the incidence rate is continually rising (1). According to the report of Cancer Surveillance Center of China (2), the prostate cancer (Pca), in China, has been occupying the second place in urologic malignancy, which has become a danger to men's health, and its incidence rate is also increasing year after year with generalizing the prostate specific antigen (PSA) screening. Although widespread PSA screening can increase the detection rate and reduce the mortality rate, urologist constantly wonders whether PSA screening with low positive rate (25%) resulted in over-treatment (3). Many factors have an influence on PSA level, including age, prostate volume (PV), prostatitis, digital rectal examination (DRE) and so on (4-7). Pca screening is testing the serum concentration of PSA in the blood. So, the different blood volume that is related with weight possesses different total mass of PSA in blood, then we can derive that weight maybe is a factor influencing the PSA level, too.
In conclusion, these factors, including age, weight and PV, should be taken into account in screening processes of Pca. Furthermore, there are reports suggested multi-parametric MRI (MP-MRI) combined PSA level or PSA density (PSAD) can increase the positive rate for Pca detection compared with PSA level and PSAD alone, but it didn’t involve age, weight and PV (8,9). The imbalanced development of economy and the diverse medical care system between developed and developing region resulted in different medical plans for the same disease in China. Moreover, some counties and municipal hospitals were not equipped with MP-MRI equipment. In this background, using MP-MRI equipment to screen Pca can bring tremendous economic burden on people and government in an underdeveloped region, and it is also not accord with the current situation of a developing country. Therefore, we are urgent to look for some new ways, which not only can reduce the false positive rate from relying on PSA screening alone but also can be more effective and economical.
Methods
Patients
Eight hundred thirty patients who underwent TURS guided prostate biopsy because of the clinical suspicion of Pca stemmed from either abnormal finding on DRE and MRI or elevated levels of PSA between January 2010 and December 2016 were retrospectively analyzed, which identified 777 patients who were biopsy naive and underwent PSA-based Pca screening and yielded 302 patients who have MP-MRI examination in addition to above tests before entry into this protocol. All patients enrolled had a serum PSA assessment, DRE and parts of patients underwent prostate imaging with MP-MRI as previously described (10). Patients with suspicion of Pca subsequently underwent trans-rectal ultrasound (TRUS) guided 12+ X-cores biopsy, what were taken from the right and left peripheral zones as previously described by Presti (11,12).
Establishment of integrated formulas
First, the indexes that were most commonly used in the clinic were chosen by the experienced chief physician and professor from our institute. Second, multivariate logistic regression analysis was used to filter these indexes in order to retain some indexes that had statistical significance and were the important independent risk factors for detecting Pca, otherwise, the indexes were excluded from our research. Third, binary logistic regression analysis was used to establish the detecting formulas. According to the presence or absence of MP-MRI, the research was divided into two parts (A and B). Seven hundred seventy seven patients entered into the research plan A, which take age, weight, PV and PSA level/PSAD into consideration. Three hundred and two patients with MP-MRI examination entered into the research plan B that takes age, weight, PV, PSA level/PSAD and MP-MRI finding into account.
Data collection and statistical analysis
MP-MRI that used Prostate Imaging Reporting and Data System (PI-RADS) (Version 2) to evaluate the probability of suffering from Pca identified the suspicious lesions based on previously established characteristics on each imaging parameter (13). For the purpose of this retrospective study B, patients with lesions visible and suspicious on T2-weighted (T2w), diffusion-weighted imaging (DWI) or dynamic contrast material enhanced (DCE) were regarded as MP-MRI screen-positive lesions (SPL) and used PI-RADS V2 to evaluate the probability of suffering from Pca with scores from 1 to 5. Patient demographics, MRI findings, and biopsy pathology correlating to 12+ X-core biopsy were collected and entered into a Microsoft Excel Version 2010. Then, the data were transferred to the Statistical Package for Social Science (SPSS) Version 10.01 for data analysis.
The parameters of composite formulas were generated by the logistic regression. To be specific, a generalized liner model of the form: ln[(P/(1−P)] = aX1 + bX2 + cX3 + dX4 + eX5 + f where a, b, c, d, e and f are the constants stemmed from the logistic regression analysis and the probability of Pca was recorded as P. In order to simplify formulas, the conversion formulas were re-written as {ln[P/(1−P)]-f}/a = X1 + b/aX2 + c/aX3 + d/aX4 + e/aX5 where X1 to X5 are five of the six variables of PSA, PSAD, age, weight, MP-MRI and PV as appropriate. The maximal point of sensitivity and specificity was used to determine the optimal threshold. Decision curve analysis was also used to compare the net benefit of the different logistic regression models generated using R code made available by Vickers (14,15).
Statistical comparisons of categorical and continuous variables were performed using Chi-square test/McNamara’s test and paired, two-tailed Student’s t-tests, respectively. For the detection of Pca, the study calculated the sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV) and overall accuracy based on thresholds of PSA >4 ng/mL, PSAD >0.15 ng/mL/mL, A >72, MRI-findings >1 and B >166. This research regarded the variables of PSA level, PSAD, A, MP-MRI and B as diagnostic instruments and used receiver operating characteristic (ROC) curves to assess the discriminative ability of pre-biopsy variables. We used binomial parametric estimation method to calculate the area under the curve (AUC) for each ROC curve by SPSS. The statistical significance between the AUC for two curves was tested by DeLong’s test. For all remaining statistical analysis, we used the SPSS (Version 10.01) to analyze.
Results
Patient demographics
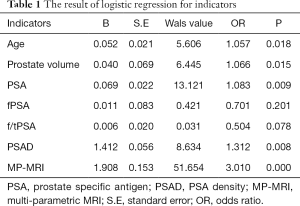
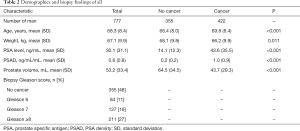
All patients haven’t been a history of biopsy. The indexes of age, PSA, PSAD, weight, PV and MP-MRI were retained by the logistic regression because these were the important independent risk factors for detecting Pca (Table 1). For research A, the mean (SD, range) age, PSA level and PSAD were 68.3 (8.4, 41–92), 30.1 (31.1, 1.1–100) and 0.6 (0.8, 0.01–3.7), respectively. For research B, the mean (SD, range) age, PSA level and PSAD were 69.3 (8.2, 43–92), 28.8 (31.0, 1.1–100) and 0.6 (0.7, 0.01–2.9), respectively (Tables 2,3). The men with Pca identified by biopsy pathology tended to be older, lighter, smaller volume of prostate, more nodules with heterogeneous signal on MP-MRI and higher PSA level/PSAD.
Full table
Full table
Full table
Establishment of integrated formulas and threshold
For the models combined with age, weight, prostate volume, PSA, PSAD and MRI findings, we, based on the logistic regression equation to maximize the sum of sensitivity and specificity for Pca detection, derived the following equations:
- For Composite A: PSA + 0.9× Age + 0.2× Weight −0.5 × Volume >72
- For Composite B: 32.1× PSAD + Age + 0.5× Weight + 32.8× MRI − 0.3× Volume >166
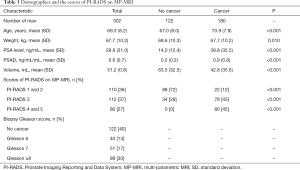
As above description, our study as consistent as previous study that indicated MP-MRI had obvious advantages compared with PSA test alone (9,16). The combined use of MP-MRI and four independent variables of research A can obtain better results getting rid of consideration of economic condition. Our study includes three MP-MRI prostate examination findings, 1 corresponds to the PI-RADS scores 1 and 2, 2 corresponds the PI-RADS scores 3 and 3 corresponds the PI-RADS scores 4 and 5. There was a statistically significant difference between 1 and ≥1, P<0.001, and between 2 and 3, P<0.001. Patients with PI-RADS 4 and 5 on MP-MRI were all diagnosed as cancer relying on pathology of biopsy in this study (Table 3). The optimal threshold of MP-MRI was >1, which means it would be considered as a positive test, if the value of MP-MRI was >1.
Comparison of PBRS and other screening methods
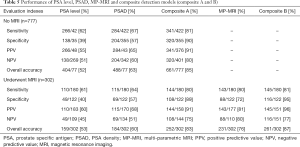
The five variables’ ROC curve was generated and the AUC were calculated (Figure 1). The Figure 1B suggested the AUC of these test range from 0.72 for PSA and 0.70 for PSAD to 0.86 for MP-MRI. There was a statistically significant difference between the AUCs for PSA level and MP-MRI, P<0.001, and for PSAD and MP-MRI, P<0.001, but not for PSA and PSAD, P=0.30. Table 4 is showing that the AUCs were compared with each other by Z-test. For the sum of sensitivity and specificity (Youden index), composite A (1.68) is significantly greater than PSA and PSAD (0.97 and 1.24, respectively), P<0.001. Moreover, there was a statistically significant difference between composite A and PSA/PSAD alone for the NPV, PPV and overall accuracy, P<0.001 (Table 5).


Full table
Full table
The sensitivity, specificity, PPV, NPV, and overall accuracy of PSA level, PSAD, A, MP-MRI and B for the detection of Pca were calculated (Table 5, Figure 2A). Testing individual measures for Pca detection (PSA level, PSAD, and MP-MRI alone), the MP-MRI was noted to have the highest sensitivity (80%), specificity (72%), PPV (82%), NPV (81%), and overall accuracy (76%). PSA had a lowest overall accuracy (43%). Composite B generated a highest specificity (95%), PPV (96%), NPV (77%), and overall accuracy (87%). Whatever the screening instruments is composite A or composite B, it has better value than PSA and PSAD alone to predict Pca. All above data is represented on the Table 5.

The prognosis of patients with Pca importantly associated with Gleason score, and there was significantly different between the biopsy Gleason score ≥7 and the biopsy Gleason score <7 for survival (17,18). The cohort of research was divided into two groups, which are test-negative and test-positive groups generated by the application of each model with the optimum thresholds (PSAD <0.15 vs. ≥0.15 ng/mL2, etc.). Figure 2B shows the distribution of biopsy Gleason score in the test-negative population as identified by the different models. The proportion of biopsy Gleason score ≥8 cancers declined from 18.4% and 15.9% (PSA and PSAD, respectively) to 6.4% and 7.9% (MP-MRI and B, respectively) in test-negative group. The MP-MRI led to the greatest proportion of men with no Pca exactly classified as screen negative while also minimizing the proportion of biopsy Gleason score ≥7 cancers that were missed. Figure 2C shows the distribution of the biopsy Gleason score in those considered ‘test-positive’ groups. Composite B not only obtained the highest proportion of men with Pca exactly categorized as screen positive but also minimized the number of men with no cancer. As a whole, the tests combined MP-MRI (MP-MRI alone or composite B) acquired highest performance in excluding men with no cancer not only higher than PSA or PSAD alone but also higher than composite A. The composite A and composite B had best effect on detecting Pca while the PSA alone had lowest effect on excluding men with no cancer, which can lead you to choice the most suitable screen measures according to the condition of patients economy and the hospital equipment.
Decision curve analysis that consists of the so-called net benefit was employed to evaluate the overall utility of these prediction models (Figure 1C). The composite B had greater net benefit vs. any one modality alone. PSA level and PSAD, compared with an approach to biopsy everyone, were of no additional net benefit until the threshold probability was close to 42%. In contrast, the composite B showed benefit in even low-risk populations with threshold probabilities for concern of Pca close to 10% and the remaining screening methods close to 18%. In addition, composite B had the best benefit whatever the threshold probability.
Discussion
Although widespread using PSA to screen the Pca has led to increasing early-stage, localized Pca detection with a controversial effect on mortality, the cost-effectiveness is worth deep-thinking in terms of low diagnostic sensitivity and specificity (19). Some specialist began to against PSA screening in all men (20). So, it is about time that specialist take significant efforts to develop other novel screening approaches with the aim of minimizing unnecessary biopsies and reducing over-treatment. Our research comprehensively considers the factors that may be influencing the PSA level, thereby; it revealed excellent performance than PSA level alone in detecting Pca.
Previous researches had identified that MP-MRI has satisfying effect on detecting Pca and the PI-RADS V2 is a better method to provide explicit criteria for assigning scores on a scale of 1–5 to stratify the level of suspicion for clinically significant cancer for each sequence routinely included within a multi-parametric prostate MR imaging examination (8,13). Based on previous study experiment of using MRI to screen Pca (8,9), we derived composite formulas A and B combined five factors including age, weight, MP-MRI finding, PSA level and PSAD. The composite A and B have higher screening evaluation index (sensitivity, etc.). The false positive rate of PSA is 9.8 times as high as the composite A, and the PPV of PSAD range from 68% to 91% for A, which can avoid more patients to biopsy and save a lot of medical resources. Based on the independent variables of composite A, the composite B added a new independent variable of MP-MRI examination. Our study revealed that composite B not only better than PSA and PSAD but also better than composite A in some ways. For example, compared with PSA (43.2%) and A (12.2%), composite B (6.6%) decreased the false positive rate by 6.5- and 1.8-fold, respectively, and the PPV and NPV of PSA ranged from 52% and 15% to 96% and 77% for B, etc.
However, composite B has its own limitations relative to composite A. MP-MRI examination, the one of component of composite B, is important difference between composite B and A, which decides the different crowds to choose a suitable screening method. Time and cost, especially in a developing region, are distinct challenges to implement the examination of MP-MRI. There is largely developmental gap between developed and developing region and also between city and country so that the doctor of developing region has to take economic question into account. Then the overall performance of composite A is not better than composite B, but it can be adopted to screen Pca for appropriate men who located on underdeveloped region and lived in poverty. Based on our study finding that composite A and B outperform PSA and PSAD alone in detecting Pca, we suggest that composite B could be applied to screen Pca for men who don't worry about economic question otherwise composite A could be used prior to PSA and PSAD.
Finally, there are lots of limitations for our study. First, the research’s cohort is not a true screening population but rather is a population that is repeatedly enriched for pre-test degree of Pca suspicion principally based elevated PSA levels, which resulted in the evaluation index (largely sensitivity) on the high side compared previous its kind. Second, on account of special Chinese national conditions, the men with suspicion of Pca identified by MP-MRI account for small proportion in all screening methods so that it could reduce the weight coefficient of MP-MRI in assessing the effect on detection for Pca. Third, some people that PSA level located on gray area (PSA level range from 4 to 10 ng/mL) were reluctant to further determine the character by biopsy but rather to choice follow-up observation, which make the proportion of these people decline consequently increasing the weight coefficient of PSA. Last, the men with Pca was diagnosed by biopsy pathology and the pathological specimen was obtained by TRUS guided biopsy that also exists false positive and false negative, so biopsy itself error can also cause our study’s result bias (10,21). In future, multi-center, large-scale and long-term screening trials will be ultimately utilized to identify our findings and define the applicability of composite A and B in Pca detection in populations of men who are not enriched by PSA-based clinical suspicion. In addition, the cost-effectiveness of using composite B to screen Pca should be evaluated in future.
Conclusions
Composite formulas combined age, weight, prostate volume, MP-MRI, PSA and PSAD have better value than PSA level and PSAD in the detection of Pca. In addition, composite A outperformed the combination of PSA/PSAD and MRI, but less than composite B, which can make the clinician choose the most appropriate screening methods to predict Pca before biopsy depending on the economic condition of men with suspicion of Pca.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No. 81630019, 81370856, 81500576), the innovation program of Science and Technology Bureau of Hefei city (81572350) and Clinical Key Subjects Program of the Ministry of Public Health (Urology).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.11.17). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by Ethics committees regarding Human Research of the First Affiliated Hospital of Anhui Medical University (PJ-20170906). Informed consent was waived. All patients involved in this study were in accordance with the ethical standards of the First Affiliated Hospital of Anhui Medical University and with the 1964 Helsinki declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Han S, Zhang S, Chen W, et al. Analysis of the status and trends of prostate cancer incidence in China. Chin Clin Oncol 2013;77:330-4.
- Andriole GL, Crawford ED, Grubb RL 3rd, et al. Prostate cancer screening in the randomized prostate, lung, colorectal, and ovarian cancer screening trial: mortality results after 13 years of follow-up. J Natl Cancer Inst 2012;104:125-32. [Crossref] [PubMed]
- Reissigl A, Pointner J, Horninger W, et al. Comparison of different prostate-specific antigen cutpoints for early detection of prostate cancer: results of a large screening study. Urology 1995;46:662-5. [Crossref] [PubMed]
- Ikuerowo SO, Ajala MO, Abolarinwa AA, et al. Age-specific serum prostate specific antigen ranges among apparently healthy nigerian men without clinical evidence of prostate cancer. Niger J Surg 2016;22:5-8. [Crossref] [PubMed]
- Gupta A, Gupta D, Raizada A, et al. A hospital based study on reference range of serum prostate specific antigen levels. Indian J Med Res 2014;140:507-12. [PubMed]
- Liu X, Wang J, Zhang SX, et al. Reference ranges of age-related prostate-specific antigen in men without cancer from Beijing area. Iran J Public Health 2013;42:1216-22. [PubMed]
- Ten Haaf K, Jeon J, Tammemägi MC, et al. Risk prediction models for selection of lung cancer screening candidates: A retrospective validation study. PLoS Med 2017;14:e1002277 [Crossref] [PubMed]
- Cerantola Y, Dragomir A, Tanguay S, et al. Cost-effectiveness of multiparametric magnetic resonance imaging and targeted biopsy in diagnosing prostate cancer. Urol Oncol 2016;34:119.e1-9. [Crossref] [PubMed]
- Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol 2011;186:1281-5. [Crossref] [PubMed]
- Isbarn H, Briganti A, De Visschere PJ, et al. Systematic ultrasound-guided saturation and template biopsy of the prostate: indications and advantages of extended sampling. Arch Esp Urol 2015;68:296-306. [PubMed]
- Presti JC Jr, Chang JJ, Bhargava V, et al. The optimal systematic prostate biopsy scheme should include 8 rather than 6 biopsies: results of a prospective clinical trial. J Urol 2000;163:163-6. [Crossref] [PubMed]
- Rosenkrantz AB, Ginocchio LA, Cornfeld D, et al. Interobserver Reproducibility of the PI-RADS Version 2 Lexicon: A Multicenter Study of Six Experienced Prostate Radiologists. Radiology 2016;280:793-804. [Crossref] [PubMed]
- Vickers AJ, Cronin AM, Elkin EB, et al. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak 2008;8:53. [Crossref] [PubMed]
- Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making 2006;26:565-74. [Crossref] [PubMed]
- Rais-Bahrami S, Siddiqui MM, Vourganti S, et al. Diagnostic value of biparametric magnetic resonance imaging (MRI) as an adjunct to prostate-specific antigen (PSA)-based detection of prostate cancer in men without prior biopsies. BJU Int 2015;115:381-8. [Crossref] [PubMed]
- Haas GP, Delongchamps N, Brawley OW, et al. The worldwide epidemiology of prostate cancer: perspectives from autopsy studies. Can J Urol 2008;15:3866-71. [PubMed]
- Brawer MK. Bicalutamide as immediate therapy either alone or as adjuvant to standard care of patients with localized or locally advanced prostate cancer: first analysis of the Early Prostate Cancer Program. BJU Int 2003;91:465-6. [Crossref] [PubMed]
- Derweesh IH, Kupelian PA, Zippe C, et al. Continuing trends in pathological stage migration in radical prostatectomy specimens. Urol Oncol 2004;22:300-6. [Crossref] [PubMed]
- Zargar H, van den Bergh R, Moon D, et al. The impact of the United States Preventive Services Task Force (USPTSTF) recommendations against prostate-specific antigen (PSA) testing on PSA testing in Australia. BJU Int 2017;119:110-5. [PubMed]
- Nafie S, Wanis M, Khan M. The efficacy of transrectal ultrasound guided biopsy versus transperineal template biopsy of the prostate in diagnosing prostate cancer in men with previous negative transrectal Ultrasound Guided Biopsy. Urol J 2017;14:3008-12. [PubMed]


