
Immune checkpoint inhibition in small cell lung cancer: a key to reach an unmet need?
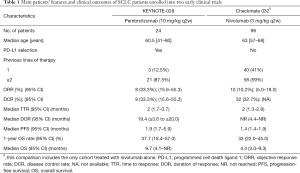
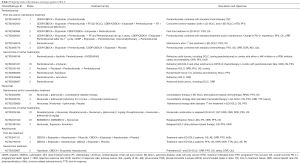
Small cell lung cancer (SCLC), accounting for 13–15% of all lung cancers, is strongly correlated with smoking and it is associated with a poor overall survival (OS) (2-year OS rate: 5%), particularly in patients with extensive disease (1,2). Furthermore, SCLC is one of the solid tumors with a higher mutational burden and an almost universal inactivation of TP53 and Rb1 genes; conversely, potential druggable genome aberrations were very rare (3,4). In the last 30 years, no new effective treatment strategies have emerged and platinum-based chemotherapy represents the standard of care in first-line setting. Despite the high objective response rates (ORR), relapse is an unavoidable event. Recommended salvage second-line chemotherapy is limited to intravenous topotecan and its efficacy depends on the duration of response (DOR) to first-line treatment (5). A treatment-free interval (TFI) shorter than 60 days was identified as a cut-off able to predict patients refractory to second-line chemotherapy and with a poor prognosis (6). Amrubicin is an alternative option in second-line setting, although it is approved only in Japan (7). In contrast to recent milestone changes in the landscape of advanced non-small cell lung cancer (NSCLC) treatment, obtained with the advent of targeted therapies and immune checkpoint inhibitors, SCLC still lacks new effective treatment strategies. In this daunting scenario, Ott and colleagues explored safety and efficacy of pembrolizumab, a highly selective anti-programmed cell death 1 (PD-1) humanized monoclonal IgG4 antibody, in patients with PD-L1 (programmed cell death ligand 1) positive extensive-stage SCLC (8). In this multi-cohort, phase Ib open-label study, 163 patients were screened and only 46 (31.7%) had tumor expressing PD-L1, with a membranous PD-L1 staining cut-off of ≥1% in tumor and associated inflammatory cells or in stroma, evaluated in a central laboratory by using the 22C3 antibody. The primary end points were safety, tolerability and ORR; secondary end-points were progression-free survival (PFS), DOR and OS. Out of 46 patients, only 24 patients were treated with pembrolizumab 10 mg/kg intravenously every 2 weeks for 24 months or until disease progression, unacceptable toxicity or other reasons. Of note, 21 of enrolled patients (87.5%) received ≥2 prior lines of therapy for advanced disease, and 9 of them (37.5%) were heavily pretreated (≥3 lines). All patients were evaluable for tolerability and safety results showed no unexpected adverse events (AEs); most common AEs of any grade were arthralgia, asthemnia and rash, while grade 3 to 5 AEs occurred in 8 patients (33.3%). One patient died due to colitis and mesenteric ischemia, and this event was supposed to be treatment-related. Regarding efficacy, twenty-two patients were evaluable for response. ORR was obtained in 8 patients (33.3%), achieving partial response in 7 patients (29.2%) and complete response in 1 patient (4.2%). Treatment responses were rapid and durable (Table 1). After a median follow-up duration of 9.8 months, median PFS was 1.9 months and median OS was 9.7 months. Noteworthy, the 6- and 12-month OS rates were 66.0% and 37.7%, respectively. In this trial, pembrolizumab showed an expected good safety profile and a clinically meaningful antitumor activity; however, some considerations have to be reported. Regarding selection criteria, KEYNOTE-028 enrolled only patients whose tumors were positive for PD-L1, with a cut-off value of ≥1%. The proportion of PD-L1 positive SCLC (31.7%) was much lower than that reported for PD-L1 positive NSCLC (66.4%) (9). Although the role of PD-L1 selection is currently unknown in SCLC, PD-L1 positive tumors seems to derive a clinically meaningful benefit from anti-PD-1 treatment (9,10); this selection may have contributed to observing the positive results of pembrolizumab in these patients, even if responses were also reported in PD-L1 negative SCLC ones receiving nivolumab (11). Despite the small sample size and the limits of a phase Ib trial, these results are particularly encouraging, considering the heavily pretreated population enrolled into this study, and they could open the way to promising treatment perspectives for an aggressive and “orphan” disease. The authors pointed out how pembrolizumab had a higher activity when compared to historical results obtained with topotecan (12). Nevertheless, taking into account the limits of an indirect comparison, ORR achieved with pembrolizumab in the intent-to-treat (ITT) population is consistent with that observed with topotecan in second-line for the treatment of sensitive relapsed SCLC patients (37.8%) (12); conversely, ORR obtained with topotecan in refractory patients was very disappointing (6.4%) and a comparison with pembrolizumab in KEYNOTE-028 is not possible due to the lack of stratification per platinum-sensitivity, a known criterion for relapsed SCLC outcome prediction (2,6). Furthermore, being a heavily pretreated population, it is our opinion that patients enrolled into this study were positively selected for good performance status (PS 0–1) and low incidence of brain metastases. Nevertheless, no data on TFI, presence of liver metastases, patients’ features (haemoglobin, albumin and sodium levels), all known to be prognostic factors (6), have been reported. This patient’s population is unlikely to reflect what we treat in real world. Responses were also durable (median 19.4 months), as typically observed with immune checkpoint inhibitors, and appeared to be longer than those obtained with topotecan (7.6 months) (12). As reported above, other immune checkpoint inhibitors have been previously tested in SCLC. Nivolumab, a fully human IgG4 monoclonal antibody directed against PD-1, has been investigated in recurrent SCLC patients, both in monotherapy and in combination with ipilimumab, in a phase I/II trial (Checkmate 032) (11). Patients received nivolumab 3 mg/kg every 2 weeks, or different schedules including nivolumab plus ipilimumab. In this study, PD-L1 status was not a selection criterion; PD-L1 expression was assessable in 69% of all patient samples and only 25 patients (17%) had tumors positive for PD-L1 (cut-off value ≥1%). Table 1 shows clinical outcomes reported in Checkmate 032 and a comparison with KEYNOTE 028. Ten out of 98 patients (10%) achieved an objective response when treated with nivolumab alone. In this cohort, median PFS and OS were 1.4 and 4.4 months, respectively. Of note, PD-L1 negativity did not preclude a response to immunotherapy. In fact, in a preplanned analysis, objective responses were achieved irrespective of PD-L1 expression, reaching 11% and 10% in platinum-sensitive and -resistant patients, respectively. Considering these results, nivolumab seems to be less active than pembrolizumab in this setting. The high proportion of PD-L1 negative patients enrolled in this study (57.3%) may have influenced results, but these considerations need further confirmation. Nivolumab as single agent was well tolerated with a toxicity profile as expected. The most common nivolumab-related toxicities of any grade were fatigue, pruritus, diarrhea, nausea and decreased appetite. Any treatment-related AE of grade 3–4 occurred in 13 (13%) patients. No treatment-related death was reported. In conclusion, it is possible that immunotherapy may have a role in the next future for the treatment of advanced SCLC, but these promising results need to be confirmed by future larger randomized trials addressed to establish the right setting and timing respect to chemotherapy and radiotherapy. In this context, different immune-oncology agents, including pembrolizumab, are currently being investigated and are reported in Table 2. Finally, the identification of potential predictive biomarkers, other than PD-L1, represents a challenge in translational research, in order to better select patients that could benefit from these novel agents.
Full table
Full table
Acknowledgments
Funding: This work was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan Grant IG 201619026.
Footnote
Provenance and Peer Review: TThis article was commissioned and reviewed by Section Editor Wei Xu, MD (Division of Respiratory Disease, Department of Geriatrics, the First Affiliated Hospital of Nanjing Medical University, Nanjing, China).
Conflicts of Interest: A Ardizzoni received honoraria for the partecipation to advisory boards and/or conferences from MSD, BMS, Pfizer, Eli-Lilly, Bayer, Boehringer. The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539-44. [Crossref] [PubMed]
- van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet 2011;378:1741-55. [Crossref] [PubMed]
- George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47-53. [Crossref] [PubMed]
- Gelsomino F, Rossi G, Tiseo M. MET and Small-Cell Lung Cancer. Cancers (Basel) 2014;6:2100-15. [Crossref] [PubMed]
- Lo Russo G, Macerelli M, Platania M, et al. Small-Cell Lung Cancer: Clinical Management and Unmet Needs New Perspectives for an Old Problem. Curr Drug Targets 2017;18:341-62. [Crossref] [PubMed]
- Ardizzoni A, Tiseo M, Boni L. Validation of standard definition of sensitive versus refractory relapsed small cell lung cancer: a pooled analysis of topotecan second-line trials. Eur J Cancer 2014;50:2211-8. [Crossref] [PubMed]
- Okuma HS, Horinouchi H, Kitahara S, et al. Comparison of Amrubicin and Weekly Cisplatin/Etoposide/Irinotecan in Patients With Relapsed Small-cell Lung Cancer. Clin Lung Cancer 2017;18:234-40.e2. [Crossref] [PubMed]
- Ott PA, Elez E, Hiret S, et al. Pembrolizumab in Patients With Extensive-Stage Small-Cell Lung Cancer: Results From the Phase Ib KEYNOTE-028 Study. J Clin Oncol 2017;JCO2017725069 [Epub ahead of print]. [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883-95. [Crossref] [PubMed]
- Ardizzoni A, Hansen H, Dombernowsky P, et al. Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: a phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative Group. J Clin Oncol 1997;15:2090-6. [Crossref] [PubMed]


