
Evaluation of clinical efficacy after combinational treatment of esophageal cancer using target artery perfusion of verapamil and chemotherapy
Introduction
Esophageal cancer (EC) is a kind of malignant tumor originating from esophageal epithelial cells. In recent years, the incidence and mortality of EC have increased, with about 456,000 new cases and about 400,000 deaths each year around the world (1,2). Most patients have already lost their chances of radical surgery when diagnosed with advanced stage EC (3,4). Besides surgery, radiation and chemotherapy are the main treatments; however, their effects are very limited due to the resistance of EC cells to these treatments (5,6). The 5-year survival of advanced EC is 10–25% because of the poor prognosis (7,8). Most of the deaths are within the first year of diagnosis (9,10). Therefore, an effective treatment for advanced EC is urgently needed.
Target artery infusion chemotherapy can form a high concentration of the drug in the lesion tissue to improve the killing effect on tumor cells (11). Tanohata first successfully used this approach in treating EC in 1977 (12). The target artery infusion chemotherapy was effective, but the 1-year survival rate was only 62.6% (13,14), which was mainly due to the resistance of tumor cells to chemotherapeutic drugs (15).
Verapamil can reverse the multidrug resistance (MDR) of tumor cells with a concentration of 6.0–10.0 µmol/L (16). However, verapamil concentration of more than 1.0–2.0 µmol/L can decrease heart rate and blood pressure and cause atrioventricular block and other cardiovascular adverse reactions. Animal experiments indicated that the concentration of verapamil in the local tissue was 50–100 times higher than that in the peripheral blood, which reached the effective concentration of drug resistance of tumor cells and did not cause significant adverse cardiovascular effects (17). Our previous studies have demonstrated that the application of target artery perfusion of verapamil combined with chemotherapy drugs significantly improved the clinical benefits in patients with liver cancer (18), colorectal cancer (19), gastric cancer (20), lung cancer (21) and malignant ascites (22). No complication of adverse cardiovascular event was reported.
In this study, we intended to investigate the clinical efficacy of verapamil combined with chemotherapy drugs in treating advanced EC using target artery infusion.
Methods
Patient enrollment
This retrospective study enrolled 46 patients with advanced EC who attended our hospital from December 31, 2009 to December 31, 2012. All patients were followed up for up to 3 years postoperation. Informed consent was obtained from all patients and the study was approved by the ethics committee of the hospital.
The inclusion criteria were as follows: (I) Karnofsky performance score (KPS) was greater than or equal to 80; (II) patients aged between 18 and 80 years; (III) expected survival time was more than 3 months; (IV) heart rate was more than 60 times/min; (V) patients voluntarily enrolled this study for treatment and signed an informed consent; (VI) esophageal barium X-ray, esophageal endoscopy, computed tomography (CT) examination, and pathological examination were carried out to confirm the diagnosis of EC; (VII) patients had no contraindication for verapamil, and the catheter could be used to support the artery in the malignant tumor and assess the efficacy of the application.
The exclusion criteria were as follows: (I) pregnant and lactating women; (II) patients with psychosis and mental agenesis; (III) patients with acute infection; (IV) allergic constitutions; (V) white blood cell (WBC) count less than 4.0×109/L, blood progenitor cells less than 10.0×109/L, and hemoglobin less than 60 g/L; (VI) patients with abnormal blood coagulation; (VII) lesions found by lateral radiography in cervical and upper thoracic regions of the esophagus; (VIII) patients did not strictly comply the treatment program; (IX) patients did not complete the course, or patients with incomplete data; and (X) patients with contraindication against cisplatin, lobaplatin, and 5-fluorouracil.
Combinational treatment of target artery perfusion of verapamil and chemotherapy
Two to four courses of the interventional therapy were given to each patient once a month. The efficacy was assessed at the fourth week after the end of the interventional therapy. The intervention was conducted by Seldinger’s technique with femoral artery catheterization. The target blood supply artery of the corresponding segment was selected according to the location of the EC (23): (I) intubation of the bilateral thyrocervical trunk artery was carried out in the cervical segment; (II) intubation of the bronchial artery and intrinsic artery of the esophagus was performed in the thoracic region; (III) intubation of the subclavian artery-inferior thyroid artery was carried out in the tumor lesions of the aortic arch of the upper thoracic region; and (IV) intubation of the left inferior phrenic artery and left gastric artery was performed in the tumor lesions of the lower thoracic region near the septum.
The combinational treatment applied was verapamil (25 mg) + chemotherapeutics (cisplatin/lobaplatin + 5-fluorouracil). Patients were administered intravenously with 100 mL of 0.9% normal saline (NS) + 0.25 mg Neothylline + 5 mg dexamethasone and 100 mL of 0.9% NS + 8–16 mg ondansetron. The total dose of chemotherapeutics (cisplatin/lobaplatin + 5-fluorouracil) was calculated based on the body weight and surface area of the patients, and the dosage of interventional therapy was allocated according to the general condition and the heart, liver, and kidney function of patients. The order of application of drugs was as follows: 50 mL of 0.9% NS + verapamil 15 mg; 50 mL of 0.9% NS + cisplatin 70–90 mg/lobaplatin 40–50 mg; 100 mL of 0.9% NS + 5-fluorouracil 1.50–1.75 g; 50 mL of 0.9% NS + verapamil 10–15 mg. Also, routine supportive and symptomatic treatments were provided. After 2–4 courses of therapy, the clinical tumor stages were evaluated by endoscopy, CT, or 64-detector-row gemstone spectral imaging/3.0-T magnetic resonance imaging (MRI). The patients who achieved CR received 1 course of radiotherapy or surgery voluntarily; otherwise, two additional courses of treatment. Median total dose of radiotherapy of upper segment lesions was 60 Gy, median total dose of radiotherapy of middle and lower segment lesions was 55 Gy.
Clinical efficacy and adverse reactions evaluation
Blood routine test, liver and kidney function tests, and electrocardiographic examination were routinely performed before and after therapy. Esophageal barium swallow test, esophageal endoscopy, endoscopic ultrasonography, and MRI were used to evaluate the clinical stage and short-term curative effect at 1 month before treatment, and at 1 month after treatment and 2 months during the treatment (24). Adverse drug reactions, dosages of analgesic drugs, changes in symptoms, KPS, and changes in body weight were recorded. The survival time of patients was followed up.
Toxic and adverse reactions were evaluated according to the National Cancer Institute- Common Toxicity Criteria (NCI-CTC) anticancer drug toxicity classification (0–IV) standard (25). Cardiac function was evaluated according to the New York Institute of Cardiology (IV–I) standard (26). Heart rate and blood pressure changes were monitored before and after perfusion, and heart function was periodically observed.
Clinical outcome evaluation and follow-up
Clinical outcomes were determined based on the dosage of pain medication, KPS, and body weight (27). Positive clinical outcome was defined as the amount of pain medication decreased more than 50% and maintained for 4 weeks or longer; KPS increased more than 20 and was maintained for 4 weeks or longer; weight increased more than 7% and was maintained for 4 weeks or longer. Negative clinical outcome was defined as the increase in the dosage of analgesics; KPS reduction; weight loss. Any other outcome was defined as stable clinical outcome. Patients were considered to have clinical benefit when at least one parameter was positive and no negative outcomes. All patients were followed up until December 31, 2015.
Based on the criteria of the Chinese Health Ministry regulations for the therapeutic efficacy of drugs for EC and new guidelines to evaluate the response to treatment in the solid tumors (28), the clinical efficacy of EC was determined in accordance with complete remission (CR), partial remission (PR), no change (NC), and progression disease(PD). CR: X-line disappeared in the barium X-ray, lesions disappeared in the esophageal endoscopic examination, the esophagus became normal, distant metastatic lesions disappeared, and metastasis in lymph nodes completely disappeared; no signs of tumor were observed in the examination, and the efficacy continued for more than a month. PR: tumor shrank more than 50% in the barium X-ray and esophageal endoscopic examination, no new lesions appeared, and distant metastases and metastases in lymph nodes reduced by more than 50% for more than a month (3). NC: tumor shrank less than 50% in the barium X-ray and esophageal endoscopic examination, and the tumor enlarged less than 25% for more than a month (4); PD: tumor enlarged more than 25% or new lesions appeared. CR + PR was determined as the overall objective response rate.
Statistical analysis
All data was analyzed using the SPSS 16.0 software. Quantitative data were compared using independent two-sample t-test and presented as mean ± standard deviation (x ± s). χ2 test was used to analyze the difference between the composition ratio and the rate. The t-test was used to analyze the group comparisons. A P value less than 0.05 was considered statistically significant.
Results
General characteristics of patients
There were 29 males and 17 females with an average age of 60.94 years old (range, 42–78 years) enrolled in this study. Histological types of all 46 cases were squamous cell carcinoma (SCC). Among them, 2 cases had neck and clavicle lymph node metastasis (LNM), 38 cases with mediastinal LNM and 8 cases with gastric drainage LNM. A total of 15 patients had upper segment lesions, 23 middle segment lesions, and 8 lower segment lesions. According to the criteria of American Joint Committee on Cancer (AJCC) (29), clinical stages of all patients before treatment were as follows: IIIA stage (16 cases), IIIB stage (13 cases), IIIC stage (15 cases), and IV stage (2 cases) (Table 1). Eighteen patients had hoarseness caused by the mediastinal lymph node compression of recurrent laryngeal nerve before treatment.
Full table
Evaluation of clinical stages after combinational treatment
A total of 44 cases with EC III stages (A, B, and C:16, 13, and 15 cases) and 2 cases with IV stages were detected before treatment. After the treatment with verapamil combined with chemotherapeutic drugs via target artery infusion, there were 10 cases with EC IIA stages, 13 cases with IIB stages, 22 cases with III stages (A, B, and C: 7, 7, and 8 cases), and 1 case with IV stage. As summarized in Table 2, the clinical stages were significantly decreased after treatment and 45 patients received one course of radical radiotherapy and 1 patient was treated by operation.
Full table
Evaluation of clinical outcomes on EC
A total of 46 patients with EC were treated with verapamil combined with two to four courses of interventional therapy. There were a total of 2 cases of CR, 39 cases of PR, 3 cases of NC, and 2 cases of PD. The overall objective response rate (CR + PR) was 89.13%.
Clinical outcomes and survival time
EC patients treated with verapamil combined with chemotherapeutic drugs achieved better KPS, weight, and positive clinical benefit of the dosage of analgesics, which were 91.30% (42/46), 52.17% (24/46), and 66.67% (4/6), respectively. After treatment, dysphagia in 42 patients was significantly improved; among them, 5 cases with narrow type had no significant improvement. Of 18 cases with voice hoarseness caused by recurrent laryngeal nerve paralysis before therapy, 11 cases recovered normal voice pronunciation and 7 cases were completely relieved. A total of 42 patients (91.30%) survived for more than a year, 24 patients (52.17%) survived for more than 2 years, and 12 patients (26.09%) survived for more than 3 years.
Adverse effects of verapamil with chemotherapy
All patients were treated with verapamil combined with chemotherapy drugs via arterial infusion. I–II degree of adverse reactions occurred, all of which were relieved in a short period of time (Table 3). Common adverse effects including WBC count decrease, nausea and vomiting, diarrhea, alopecia, fever and liver failure were observed in 17 cases (36.96%), 23 cases (50.00%), 3 cases (6.50%), 18 cases (39.13%), 1 case (2.17%) and 3 cases (6.52%) respectively. Before and after using verapamil, the vital signs (respiration, pulse, and blood pressure) of the patients were not significantly changed and electrocardiogram did not show significant differences (Table 4).

Full table

Full table
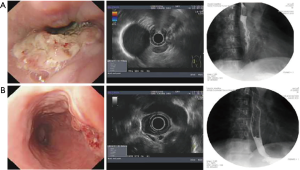
Typical case 1: the patient with EC in the middle and lower segments of the esophagus combined with mediastinal LNM had obvious obstruction and was evaluated as EC IIIA stage by CT combined with MRI and endoscopic ultrasonography (Figure 1A). The patient was treated with two courses of 25 mg verapamil combined with 1.75 g 5-fluorouracil and 50 mg lobaplatin, followed by a 2-week interventional chemotherapy. EC was evaluated as IIB stages, and the sense of obstruction disappeared (Figure 1B). The patient was successfully treated with radical surgery. Pathological examination revealed that the carcinoma tissues invaded the middle of the muscular layer after surgery, no metastasis was found in 20 dissected lymph nodes, and the pathologic stage was pT2N0M0 after operation.
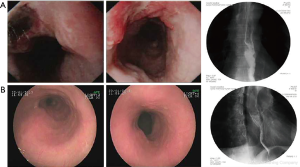
Typical case 2: for the patient with EC combined with left supraclavicular LNM in the middle segment of the esophagus, endoscopy, CT, and esophageal barium X-ray were used for detection before therapy (Figure 2A). Three courses of interventional therapy comprising 25 mg verapamil combined with 80 mg cisplatin (DDP) and 1.75 g 5-fluorouracil were given to the patient. 5-Fluorouracil (1.0 g ×3 d) was administered for intravenous chemotherapy on the second day after the interventional chemotherapy. The esophageal lesions significantly reduced after the therapy (reached CR criteria) (Figure 2B). The patient refused surgical treatment, and were treated with radical radiotherapy.
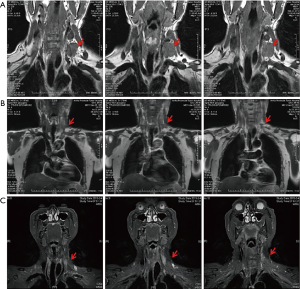
Typical case 3: this patient was diagnosed with EC combined with the left supraclavicular LNM, and the left mediastinal LNM combined with voice hoarseness caused by recurrent laryngeal nerve paralysis. Before the interventional therapy, the size of the left supraclavicular lymph nodes was about 4×3 cm2, as detected by MRI (Figure 3A). First, half of the doses of verapamil, cisplatin, and 5-fluorouracil were administered into the superficial cervical artery, the branches of the subclavian artery, and the branch of the left external carotid artery. Then, the remaining half of the doses of verapamil, cisplatin, and 5-fluorouracil were administered into the branch of the left external carotid artery and branches of the bronchial artery. Each target artery perfusion was repeated two times (Figure 3B). The total amount of drugs administered every time was 25 mg verapamil with 90 mg cisplatin and 1.75 g 5-fluorouracil. After four courses of the interventional therapy, the left clavicle lymph nodes disappeared, as revealed by MRI (Figure 3C). After four courses of chemotherapy followed by radiotherapy (the total radiation dose was 6,000 cGy), the disease-free survival at follow-up was 60 months.
Discussion
In this study, 46 patients with advanced EC were treated with verapamil combined with chemotherapeutic drugs via target artery infusion. Our results suggested that the clinical stages of patients with EC were significantly reduced with improved short-term curative effects and alleviated clinical symptoms. Patients’ survival time was prolonged which granted opportunity for radical radiotherapy and operation. Compared to Tai et al.’s study, our study achieved better 1-, 2-, and 3-year survival rates (30).
MDR is one of the main reasons that restrict the curative effect on malignant tumors in a clinical scenario (31,32). It has been shown that P-glycoprotein (P-gp) hydrolyzes adenosine triphosphate (ATP) to generate adenosine diphosphate (ADP), and energy. P-gp binds to intracellular chemotherapy drugs with the involvement of calcium ions that pump the drugs out of the cell, decreases the intracellular drug concentration and reduces the toxic effects on tumor cells. This process leads to the development of MDR (33,34). Verapamil is a calcium channel antagonist that inhibits the expression of mdrl gene and the synthesis of P-gp to increase the concentration of chemotherapeutic drugs in cancer cells and hence overcome the drug resistance of tumor cells (35). It has been demonstrated that 6–10 µmoL/L verapamil completely inhibits P-gp to reverse MDR of malignant tumor cells and increases the sensitivity of tumor cells to chemotherapeutic drugs. However, adverse reactions such as decrease of the heart rate and blood pressure, atrioventricular block etc. occurred when the verapamil concentration reached 1–2 µmol/L in the serum, which limited the intravenous application of verapamil in cancer therapy.
Results of verapamil perfusion through canine hepatic artery showed that the drug concentration in the local tissue was 5–10 times higher than that in the blood, and adverse cardiovascular effects such as decrease of heart rate, blood pressure and atrioventricular block did not occur (17). The application of target artery perfusion with verapamil combined with chemotherapeutic drugs has yielded good clinical results in treating primary liver cancer, advanced colorectal cancer, advanced gastric cancer, advanced lung cancer, and malignant ascites (18-22).-Hence, the same interventional therapy was used in treating patients with advanced EC. Although simple arterial infusion chemotherapy has the advantages of less side effects and good tolerance, which can effectively improve the quality of life of patients, it cannot effectively improve the overall survival time (31,32). This is a pilot study to demonstrate the effectiveness of combination verapamil and chemotherapy drugs in the treatment of EC. Further study with larger sample and control group would be conducted to further confirm our findings.
Acknowledgments
Funding: This study was supported by National Natural Science Foundation of China (81350005 and 81201576), 2012’Scientific Research Grant of Anhui Provincial Science and Technology Department (12070403058), Natural Science Foundation of Anhui Province (1408085MH211 and 08085QH182), Natural Science Research Projects of Anhui Province (KJ2013Z184); 2012’Chinese Medicine Scientific Research Project Grant of Anhui Provincial Health Department (2012zy43).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.11.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the hospital and written informed consent was obtained from all patients (No. 20090117).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Montgomery EA, Basman FT, Brenan P, et al. Oesophageal Cancer. In: Stewart BW, Wild CP. World Cancer Report 2014. World Health Organization 2014;528-43.
- Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2224-60. [Crossref] [PubMed]
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [Crossref] [PubMed]
- Horner M, Ries L, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2006, National Cancer Institute. Bethesda, MD. Available online: http://seer.cancer.gov/csr/1975_2006/
- Polee MB, Eskens FA, van der Burg ME, et al. Phase II study of bi-weekly administration of paclitaxel and cisplatin in patients with advanced oesophageal cancer. Br J Cancer 2002;86:669-73. [Crossref] [PubMed]
- Shridhar R, Almhanna K, Meredith KL, et al. Radiation therapy and esophageal cancer. Cancer Control 2013;20:97-110. [Crossref] [PubMed]
- Burtness B, Ilson D, Iqbal S, et al. New directions in perioperative management of locally advanced esophagogastric cancer. Am Soc Clin Oncol Educ Book 2014;e172-8. [Crossref] [PubMed]
- Delcambre C, Jacob JH, Pottier D, et al. Localized squamous cell cancer of the esphagus; retrospective analysis of three treatment schedules. Radiother Oncol 2001;59:195-201. [Crossref] [PubMed]
- Adamson D, Blazeby J, Nelson A, et al. Palliative radiotherapy in addition to self-expanding metal stent for improving dysphagia and survival in advanced oesophageal cancer (ROCS: Radiotherapy after Oesophageal Cancer Stenting): study protocol for a randomized controlled trial. Trials 2014;15:402. [Crossref] [PubMed]
- Mayer Robert J. Gastrointestinal Tract Cancer. In: Longo DL, Fauci AS, Kasper DL, et al. Harrison's principles of internal medicine 1 (18th ed.). New York: McGraw-Hill Medical Publishing Division, 2008:764–5.
- Polednak AP. Trends in survival for both histologic types of esophageal cancer in US surveillance, epidemiology and end results areas. Int J Cancer 2003;105:98-100. [Crossref] [PubMed]
- Tanohata S. The clinical investigation of esophageal arteriography in esophageal cancer--with special reference to properesophageal arteriography-- (author's transl). Newsl Int Coll Dent India Sect 1977;37:103-24. [PubMed]
- Suzuki T, Izumi Y, Miura A, et al. Successful management of the recurrent esophageal cancer following esophagectomy at a different time with combined local treatment of chemoradiotherapy and hepatic arterial infusion chemotherapy. Gan To Kagaku Ryoho 2008;35:1737-9. [PubMed]
- Ikebe M, Kitamura M, Saitoh G, et al. Multimodality therapy containing hepatic arterial infusion chemotherapy for liver metastasis of esophageal cancer-a case report. Gan To Kagaku Ryoho 2012;39:1555-7. [PubMed]
- Shuto K, Ohira G, Kono T, et al. Regional treatment of esophageal liver metastasis by intra-arterial low-dose 5-FU therapy. Gan To Kagaku Ryoho 2010;37:2409-11. [PubMed]
- Qiang F, Guangguo R, Yongtao H, et al. Multidrug resistance in primary tumors and metastases in patients with esophageal squamous cell carcinoma. Pathol Oncol Res 2013;19:641-8. [Crossref] [PubMed]
- Sun X, Yin Q, Chen D, et al. Determination of verapamil in dog serum and tissues by reversed-phase high performance liquid chromatography. Se Pu 2004;22:255-7. [PubMed]
- Huang J, Duan Q, Fan P, et al. Clinical evaluation of targeted arterial infusion of verapamil in the interventional chemotherapy of primary hepatocellular carcinoma. Cell Biochem Biophys 2011;59:127-32. [Crossref] [PubMed]
- Liu Y, Lu Z, Fan P, et al. Clinical efficacy of chemotherapy combined with verapamil in metastatic colorectal patients. Cell Biochem BiopHys 2011;61:393-8. [Crossref] [PubMed]
- Ning Z, Chen D, Liu A, et al. Efficacy of chemotherapy combined with targeted arterial infusion of verapamil in patients with advanced gastric cancer. Cell Biochem Biophys 2014;68:195-200. [Crossref] [PubMed]
- Huang J, Zhang T, Ma K, et al. Clinical evaluation of targeted arterial perfusion of verapamil and chemotherapeutic drugs in interventional therapy of advanced lung cancer. Cancer Chemother Pharmacol 2013;72:889-96. [Crossref] [PubMed]
- Jia W, Zhu Z, Zhang T, et al. Treatment of malignant ascites with a combination of chemotherapy drugs and intraperitoneal perfusion of verapamil. Cancer Chemother Pharmacol 2013;71:1585-90. [Crossref] [PubMed]
- Pramesh CS, Mistry RC. Surgical treatment for cancer of the oesophagus and gastric cardiain Hebei, China. Br J Surg 2004;91:511. [Crossref] [PubMed]
- Lü F, Xue Q, Shao K, et al. Preliminary experience of clinical applications of the 7th UICC-AJCC TNM staging system of esophageal carcinoma. Zhonghua Zhong Liu Za Zhi 2012;34:461-4. [PubMed]
- Xing L, Liang Y, Zhang J, et al. Definitive chemoradiotherapy with capecitabine and cisplatin for elder patients with locally advanced squamous cell esophageal cancer. J Cancer Res Clin Oncol 2014;140:867-72. [Crossref] [PubMed]
- The Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Boston, Mass: Little, Brown & Co., 1994:253-6.
- Kim JJ, Jung HC, Song IS, et al. Preopertive evaluation of the curative respectability of gastric cancer by abdominal computed tomography and ultrasonography:a prospective study. Korean J Intern Med 1997;12:1-6. [Crossref] [PubMed]
- Duffaud F, Therasse P. New guidelines to evaluate the response to treatment in solid tumors. Bull Cancer 2000;87:881-6. [PubMed]
- Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manual.7th ed. New York: Springer-Verlag 2010:101-3.
- Tai P, Yu E. Esophageal cancer management controversies: Radiation oncology point of view. World J Gastrointest Oncol 2014;6:263-74. [Crossref] [PubMed]
- Liu L, Zuo LF, Guo JW. ABCG2 gene amplification and expression in esophageal cancer cells with acquired adriamycin resistance. Mol Med Rep 2014;9:1299-304. [Crossref] [PubMed]
- Wang LS, Chow KC, Wu YC, et al. Inverse expression of dihydrodiol dehydrogenase and glutathione-S-transferase in patients with esophageal squamous cell carcinoma. Int J Cancer 2004;111:246-51. [Crossref] [PubMed]
- Xiang QF, Zhang DM, Wang JN, et al. Cabozantinib reverses multidrug resistance of human hepatoma HepG2/adr cells by modulating the function of P-glycoprotein. Liver Int 2015;35:1010-23. [Crossref] [PubMed]
- Noguchi K, Katayama K, Sugimoto Y. Human ABC transporter ABCG2/BCRP expression in chemoresistance: basic and clinical perspectives for molecular cancer therapeutics. Pharmgenomics Pers Med 2014;7:53-64. [Crossref] [PubMed]
- Rogalska A, Szwed M, Rychlik B. The connection between the toxicity of anthracyclines and their ability to modulate the P-Glycoprotein-Mediated Transport in A549, HepG2, and MCF-7 Cells. ScientificWorldJournal 2014;2014:819548 [PubMed]


