Wip1−/− ameliorates hepatic ischemia/reperfusion injury via PI3K/Akt activation
Introduction
As the largest organ of the body liver plays important roles in various biological and physiological functions including biosynthesis, secretion and transformation, etc. Ischemia/reperfusion (I/R) injury is a causal factor which contributes to both morbidity and mortality in several clinical settings, including hepatic sinusoidal obstruction syndrome, hemorrhagic shock, trauma and cardiac arrest (1). Moreover, during hepatectomy and vascular reconstruction, a major obstacle to transplantation surgery and liver resection where reperfusion after sustained ischemia is unavoidable is the vulnerability of the liver to I/R injury. Hepatic I/R injury occur in diverse pathological and clinical circumstances, including liver preservation for transplantation, liver surgery, hemorrhagic shock-resuscitation, veno-occlusive disease, and heart failure as well. The liver can present three different forms of ischemia, the cold ischemia (or hypothermic), the warm ischemia (or normothermic), and the rewarming ischemia. Cold ischemia occurs while the organ awaits implantation where it is intentionally applied to reduce metabolic activities of the graft, and thus it happens almost exclusively in transplant settings. Warm ischemia occurs when hepatic inflow occlusion (Pringle maneuver) or total vascular exclusion are induced to minimize blood loss when dividing the liver parenchyma. Therefore, unlike cold ischemia, warm ischemia occurs in diverse situations, including hepatic transplantation and trauma, hemorrhagic shock, and liver surgery. However, rewarming ischemia typically occurs during situations of graft manipulation or graft implantation or while performing the vascular reconstruction when the cold liver is subjected to room. Although ischemic stress by itself primes cells for damage and will ultimately induce cell death, ischemic injury usually does not manifest itself until after the ischemic liver is reperfused (2).
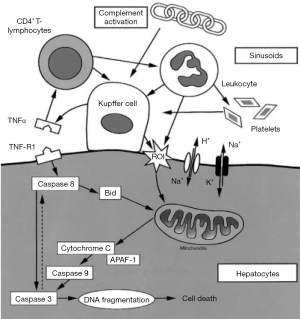
In the 1980s, it has already been shown that cold ischemia caused injury specifically to sinusoidal endothelial cells (SEC). Moreover, endothelial wall disruption induces leukocyte and platelet adhesion, the latter of which leads to SEC apoptosis after cold ischemic liver reperfusion (3-6). Unlike cold ischemia, warm ischemia is poorly tolerated and leads to rapid death of hepatocytes (7). This severe injury of liver cells is most likely preceded by massive destruction of endothelial cells (8). There is a growing body of studies focused on understanding the underlying mechanisms of warm I/R injury. The most well studied mechanism is TNF-a mediated apoptosis of the Kupffer cells. It has been found that I/R activates the resident macrophages of the liver—the Kupffer cells, and Kupffer cell activation after reperfusion generate reactive oxygen species (ROS) and leads to release of both lysosomal enzymes and various pro-inflammatory cytokines like TNF-α (9,10), whereas functional inactivation of Kupffer cells, on the contrary, attenuates liver injury during both early and late reperfusion (9,11,12). Moreover, during early stages of reperfusion, the release of oxygen free radicals as well as further binding of these cytokines to respective receptors lead to the initiation of the complex apoptotic machinery and eventually programmed cell death of hepatocytes (10). It has been proved that several pro-apoptotic proteins including caspase-8 and caspase-3 are activated during the reperfusion phase, and cytochrome c is found to be released from the mitochondria into the cytoplasm. This cascade activates both extrinsic and intrinsic apoptotic pathway, finally resulting in cell death (13) (Figure 1). In addition, stress-activated protein kinases, especially c-Jun kinase, are found activated during hypoxia reoxygenation by extracellular stimuli and results in activation of pro-apoptotic transcription factors and initiation of apoptosis (14,15).
The loss of mitochondrial ATP production is the primary ischemic stress to liver and most other solid tissues. Another mechanism of I/R injury is membrane Na/K ATPase inactivation due to ATP depletion, which leads to cell rounding and swelling, as well as mitochondria swelling and ER dilatation, and bleb formation (16,17). Increasing evidence indicate apoptosis activation as a key mechanism of hepatic I/R injury (6,8). However, the observations of post-ischemic cell swelling, vacuolation, karyolysis, and cell content release, are characteristic features of necrosis (18). Therefore, whether cells die predominantly by apoptosis or necrosis needs to be determined in experimental or clinical settings.
Autophagy, although the first known role is its action during nutrient starvation, is rapidly studied and focused on its role in liver diseases including liver I/R (19-22). Autophagy is an intracellular degradative pathway that targets different cellular components to lysosome for degradation in order to maintain cellular homeostasis and energy supply for substrates. Increasing evidence has linked autophagy to a detrimental role in hepatic I/R injury. It clears out dysfunctional or abnormal mitochondria to make sure optimal cellular function and survival advantage. However, with insufficient or impaired autophagy, damaged mitochondria were accumulated, leading to uncontrolled formation of ROS and destructive mutation of mitochondrial DNA, and ultimately cell death. Hepatic I/R injury could be attenuated by inhibition of autophagy, which is mediated partly through down-regulation of NF-κb and Akt serine/threonine phosphatase signaling pathways (23-26).
Wip1 (also known as PP2Cδ), is a nuclear serine/threonine phosphatase which is encoded by protein phosphatase magnesium-dependent 1 delta, or PPM1D. It belongs to the Ser/Thr PP2C family of phosphatases (27). Wip1 is a key regulatory protein in DNA repair pathways (28). In response to various stresses including UV, γ-radiation, and alkylating agents, wip1 is activated by MAPK and p53Jeny (29-31). It is not only a direct transcriptional target of p53 but also negatively regulates p53, thus forming a negative regulatory feedback loop (32,33). As a protein phosphatase, activated Wip1 can directly dephosphorylate various proteins including p38 MAPK, checkpoint kinases CHK1/2, NF-kB, ATM, uracil DNA glycosylase (UNG), MDM2, γ-H2AX, BAX and p53. By dephosphorylating these proteins, Wip1 functions as a key homeostatic regulator that reverses various signaling cascades and induces the damaged cells to re-enter the normal cell cycle following completion of DNA repair (32,34-42). Moreover, Wip1 is emerging as a potent regulator of tumorigenesis as it is frequently amplified/overexpressed in numerous primary human tumors, including breast, ovarian, and pancreatic cancers (43-46). Meanwhile, in addition to programmed cell death and autophagy, Wip1 also plays critical roles in multiplex cellular functions including cell proliferation and senescence, cell cycle arrest, immune-regulation and inflammatory response (47-49). It is found to be expressed in diverse immune-regulating cells like hematopoietic progenitor cells, neutrophils, macrophages and B/T lymphocytes, and hence Wip1 knock-out mice undoubtedly displays immunodeficiency and susceptibility to viral infection (50). To date, the role of Wip1 in liver I/R injury has not been studied yet. In this study, we are aimed to discover the functional role and underlying mechanism of Wip1 in acute liver injury by utilizing partial (2/3) liver warm I/R injury model.
Methods
Wip1 knock-out mice
In order to generate C57BL wip1-/- mice, 129sv genetic background wip1 knock-out mice were intercrossed with C57BL/6 genetic background wild type mice, then the 129sv/C57BL/6 wip1+/- springs were intercrossed with C57BL/6 wild type mice for 7 serial generations to get C57BL/6 wip1+/-. Finally, these C57BL6 wip1+/- mice were mated to breed C57BL6 wip1-/- and wip1+/+ littermates.
Liver injury assessment
Wip1-/- and their wild-type littermates at 10–14 weeks with the same gender were assigned as experimental group and control group respectively. A 70% hepatic warm I/R model was established and used in this study. Time of ischemia was set as 60 and 90 min. After 6 or 24 h of reperfusion, peripheral blood was collected and mice liver was harvested shortly after euthanasia. The severity of liver injury was assessed through serum ALT levels and Suzuki’s scores using H&E staining of the ischemic liver lobes. The TUNEL assay was utilized for evaluation of apoptosis.
Analysis of liver ALT enzyme
The level of serum ALT enzyme was detected with a chemical analyzer (Olympus AU1000, Tokyo, Japan).
H&E staining
Immediately after collection the liver tissues were fixed in 10% formalin for more than 24 hours, and embedded in paraffin. The tissue blocks were cut into 3-µm sections. After that, the sections were then stained with hematoxylin-eosin (HE) and the pathological variations were observed under a light microscopy. Suzuki classification was performed to evaluate the severity of I/R injury, consisting three parameters includes sinusoidal congestion, vacuolization of hepatocyte cytoplasm, and parenchymal necrosis. Suzuki’s scores were graded numerically and implicated at Table 1.

Full table
TUNEL assay
Apoptosis of histological sections were detected using TUNEL kit (Beijing Ding Guo Biological Co., Ltd., Beijing, China) according to the manufacturer’s instructions. Briefly, the sections were firstly deparaffinated and hydrated, and digested with proteinase K for 20 min. After extensive rinse with 0.01 M PBS, the sections were then incubated with terminal deoxynucleotidyl transferase and digoxigenin-dUTP at 37 °C for 1 h, followed by incubation with a biotin-conjugated anti-digoxin antibody and streptavidin-biotin complex for 30 min each. After extensive washes with PBS, the slides were immersed in DAB solution. All slides were counterstained with Mayer’s hematoxylin. Quantitative analysis is calculated as a ratio of TUNEL-positive cells versus total cells which is counted under a microscope field.
Western blot
The expression of wip1, Akt, mTOR, p70S6K, S6 and their phosphorylated protein levels were determined through western blot assay. Briefly, total proteins were extracted from the liver tissues by using appropriate cold lysis buffer based on the manufacturer’s instructions. The protein concentrations were determined by BCA method (Beijing Ding Guo Biological Co., Ltd.), following by sample loaded onto 12% SDS-PAGE, separated electrophoretically, and transferred onto a PVDF membrane. After blocking non-specific binding sites with 5% dried skim milk in TBST at room temperature for 1 h, the membrane was then incubated overnight at 4 °C with primary antibodies, followed by incubation with horseradish peroxidase-conjugated secondary antibodies (1:3,000) at room temperature for 1 h. After extensive washes, protein expressions were detected by an enhanced chemiluminescence (ECL) method, imaged using automated wash machine TL-760S (Tyrone medical equipment factory, Taixing, Jiangsu, China), and analyzed by BandScan software. The data were adjusted to correspond internal reference expression [integrated optical density (IOD) value of target protein versus IOD of correspond internal reference] in order to eliminate the variations of protein expression. All antibodies used in the study were purchased from Cell Signaling Technology (Danvers, MA, USA).
Real-time RT-PCR assay
Total RNA was extracted from the liver tissues by using RNA extraction kit (Omega, Philadelphia, PA, USA) following the manufacturer’s protocol followed by real-time RT-PCR assay to detect wip1 mRNA levels. Briefly, reverse transcription (RT-PCR) was performed by using First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions, and the level of wip1 mRNA transcription was quantified by real-time PCR with Power SYBR Green PCR Master Mix (Applied Biosystems, Waltham, MA, USA) and ABI 7500 real-time PCR System (Applied Biosystems). Meanwhile, a no-template reaction was used as a background control in parallel and the GAPDH gene was selected as the internal control in our study.
Statistical analysis
All of the data were analyzed by using GraphPad Prism statistical software and displayed as means ± SD. Differences among groups were determined by utilizing one-way ANOVA or unpaired Student’s t-test. P value <0.05 and <0.01 were considered to be statistical significant.
Results
Wip1 knock-out attenuated liver I/R injury in mice
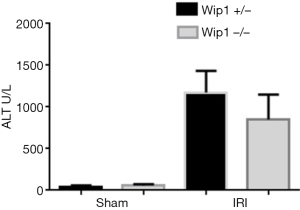
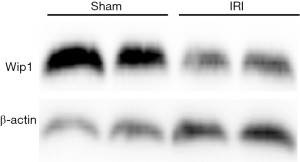
In order to investigate the role of wip1 in liver I/R injury, firstly we were interested in examining the expression level of wip1 during I/R injury in wild-type mice. As shown in Figure 2, after 60 min of ischemia and 6 h of reperfusion, wip1 expression was remarkably reduced in wild-type I/R mice compared with sham-operated mice, indicating that wip1 may be a key regulatory protein during liver injury. In addition, after 90 min of ischemia and 6 h of reperfusion, the wip1 mRNA transcription was also significantly down-regulated in wild-type mice (data not shown). Wip1 knock-out was confirmed by genotyping (data not shown). In order to determine the functional role of wip1 expression in I/R injury, we next investigated the impact of wip1-/- on liver injury by examining the possible changes of serum ALT levels. As shown in Figure 3, after 60 min of ischemia and 6 h of reperfusion, the serum levels of ALT were significantly decreased in wip1-/- mice (851.3±270.9 U/L) compared with wild-type mice (1172.5±237.1 U/L) (P<0.01), indicating that wip1 down-regulation in return may protect liver tissues against I/R injury. Moreover, there is no significant difference in serum ALT levels between wip1-/- mice (56.6±12.9 U/L) and wild-type mice (44.3±11.3 U/L) in sham operated mice (P=0.369), excluding the possibility that operation itself may leads to a decreased ALT level. Hence, we concluded that liver injury was attenuated in wip1 knock-out mice.
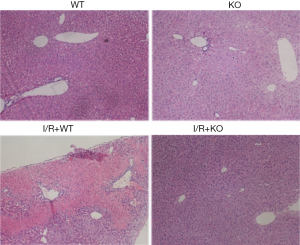
Wip1 knock-out alleviates pathological changes after I/R injury
Next, to confirm the protective role of wip1-/- in liver injury, Suzuki’s score was conducted and graded numerically to evaluate the severity of liver injury, and morphological changes in sinusoidal congestion, vacuolization of hepatocyte cytoplasm, and parenchymal necrosis were evaluated and detected by H&E staining (see Table 1).
As shown in Figure 4, after 90 min of ischemia and 24 h of reperfusion, the severity of liver morphological changes following I/R injury was significantly compromised by wip1-/-, with the Suzuki’s scores of 5.25±0.43 in wip1-/- mice compared with 7.75±0.43 in wild-type mice (P<0.01), confirming that wip1-/- may protect liver tissues against I/R injury.
Wip1-/- leaded to a decreased programmed cell death following liver I/R injury
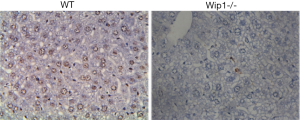
Wip1 is an important regulator in a number of critical cellular functions including proliferation, cell cycle arrest, senescence and programmed cell death, autophagy, immunoregulation as well as inflammatory response (47-49), whereas the underlying mechanisms through which wip1-/- protected against liver injury was still unknown. We hypothesized that wip1-/- may protect liver tissues from I/R injury via suppressing apoptosis. In order to test this hypothesis, TUNEL assay was performed to detect variations of in situ apoptotic cell percentage by comparing liver samples from wip1-/- mice with wild-type mice. As shown in Figure 5, after 90 min of ischemia and 24 h of reperfusion, the rate of apoptosis was unsurprisingly reduced in wip1-/- mice (30.4%±3.7%) compared to wild-type mice (62.3%±5.6%) (P<0.01), supporting our hypothesis that wip1-/- plays a protective role in liver injury by inhibition of programmed cell death.
Wip1-/- plays a protective role in liver injury via activating PI3K/Akt signaling pathway
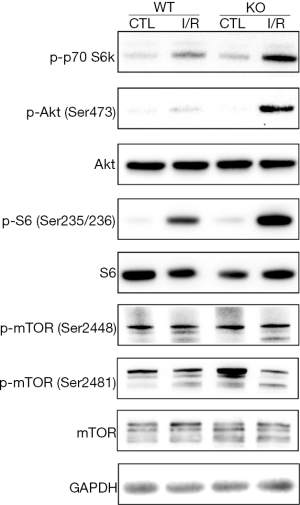
The mechanisms of liver I/R injury are complicated by various factors. It can be mediated by release of ROS, lipid peroxidation, cell apoptosis and autophagy, and many other cellular molecules and signaling pathways. Previous studies have demonstrated that wip1-/- prevented liver I/R injury by inhibiting apoptosis, yet the mechanism underlying remained unclear. It is well known that the PI3K/Akt pathway plays an important role in cellular survival and apoptosis. Therefore, we planned to investigate whether wip1-/- could activate PI3K/Akt pathway to protect liver tissues against I/R injury. The protein levels of Akt, p-Akt, mTOR and p-mTOR are shown in Figure 6 by comparing wild-type mice or wip1-/- mice with sham-operation or I/R injury. After 90 min of ischemia and 6 h of reperfusion, the protein levels of mTOR, p-mTOR (Ser2448), Akt and S6 ribosomal protein showed no significant changes while the protein levels of p-Akt (Ser473), p-p70 S6K (Thr389) and p-S6 (Ser235/236) were clearly increased in the wip1-/- mice following I/R injury compared with wild-type mice. Taken together, these results provided strong evidence that wip1-/- attenuated apoptosis at least partly by activating the PI3K/Akt pathway.
In summary, our study has revealed that wip1 expression was suppressed during hepatic I/R injury and wip1-/- in return attenuated I/R injury, suggesting a critical role of wip1 expression in liver self-regulation during I/R injury. Moreover, our study has demonstrated for the first time that wip1-/- exerted its protective effect on I/R injury through inhibiting apoptosis by at least partially activating PI3K/Akt pathway.
Discussion
Hepatic I/R injury is a complex pathological process associated with liver transplantation, shock, and trauma. The process of hepatic I/R injury is a serious threat to human health. I/R injury activate Kupffer cells which generate ROS and lead to release of lysosomal enzymes and various pro-inflammatory cytokines including TNF-α (9,10). Further binding of these cytokines to their respective receptor during early stages of reperfusion or release of oxygen free radicals is shown to initiate the complex apoptotic machinery and eventually leads to cell death of hepatocytes (10).
Wip1 (also known as PP2Cδ), is an Mg2+-dependent nuclear serine/threonine phosphatase encoded by PPM1D gene (27). Wip1 is a key regulatory protein in DNA repair pathways (28). It is activated by MAPK and p53 in response to a sort of stresses, including UV, γ-radiation, and alkylating agents (29-31). Activated Wip1 induces damaged or injured cells re-enter the normal cell cycle process by directly dephosphorylating various key proteins participating in DNA damage repair including CHK1/2, NF-kB, p38 MAPK, UNG, ATM MDM2, γ-H2AX, BAX and p53 (32,34-42), indicating its important role in regulating DNA repair pathways. In our study, we have found that wip1 expression level was significantly reduced after I/R injury, and more importantly, wip1 knock-out mice presented an ameliorated liver injury, indicating that I/R injury may down-regulate wip1 expression which in return protect liver tissues from injury. Therefore, wip1 down-regulation may serve as a self-protective regulator following liver injury.
Several pathways have been reported to associate with I/R injury, including ROS/MAPK pathway (51), ROS/JNK/Bcl-2 pathway and HMGB1/TLR4/NF-κB pathway. The PI3K/Akt pathway, a well-known cell survival pathway, plays a critical role in the regulation of cell proliferation and cell apoptosis (52). Moreover, the PI3K/Akt signaling pathway exerts a strong protective effect on I/R injury through the inhibition of apoptosis (53-56). We therefore investigated whether wip1-/- protected liver tissues against I/R injury through the activation of the PI3K/Akt pathway. To investigate the anti-apoptotic mechanism of wip1-/-, we first performed the TUNEL assay to confirm its anti-apoptotic effect after I/R injury, followed by measuring the protein levels of Akt, mTOR, S6 and their phosphorylated proteins. Our results showed that wip1-/- did not change the expression of these proteins at their baseline levels. However, I/R injury up-regulated p-Akt, p-p70 S6K and p-S6 protein levels, and wip1-/- further markedly up-regulated these protein level whereas the expression of total Akt and S6 remains unchanged. These results indicated that wip1-/- prevent apoptotic cell death probably through activating PI3K/Akt pathway.
Hepatic I/R injury involve various complex and multifactorial mechanisms and these mechanisms need to be further explored. In our present study, we investigated the protective effect of wip1-/- on hepatic I/R injury by activating PI3K/Akt pathway. However, other studies have found that Akt as well as NF-kB activation could also attenuate I/R injury by inhibition of autophagy (23-26), and whether or not wip1-/- protects liver tissues from I/R injury by inhibiting autophagy or via other mechanisms like NF-kB activation remains to be determined. In addition, as a serine/threonine protein phosphatase, wip1 can directly dephosphorylate various downstream proteins like CHK1/2, ATM and BAX to participate in a number of biological processes, particularly DNA damage response and cell cycle regulation, and thus whether wip1-/- exerts its protective effect on I/R injury through other mediators and biological functions waits to be addressed. We are currently working toward answering these questions.
Acknowledgments
Funding: This study was supported by grants from the CAMS Innovation Fund for Medical Sciences (No. 2016-I2M-1-001) and the National High-tech Research and Development Projects [863] (No. 2015AA020303).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.12.16). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Peking Union Medical College Hospital Review Board (No. PUMCH-2014-A230854).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jaeschke H. Mechanisms of reperfusion injury after warm ischemia of the liver. J Hepatobiliary Pancreat Surg 1998;5:402-8. [Crossref] [PubMed]
- Caldwell-Kenkel JC, Currin RT, Tanaka Y, et al. Reperfusion injury to endothelial cells following cold ischemic storage of rat livers. Hepatology 1989;10:292-9. [Crossref] [PubMed]
- McKeown CM, Edwards V, Phillips MJ, et al. Sinusoidal lining cell damage: the critical injury in cold preservation of liver allografts in the rat. Transplantation 1988;46:178-91. [Crossref] [PubMed]
- Otto G, Wolff H, David H. Preservation damage in liver transplantation: electron-microscopic findings. Transplant Proc 1984;16:1247-8. [PubMed]
- Clavien PA, Morgan GR, Sanabria JR, et al. Effect of cold preservation on lymphocyte adherence in the perfused rat liver. Transplantation 1991;52:412-7. [Crossref] [PubMed]
- Sindram D, Porte RJ, Hoffman MR, et al. Platelets induce sinusoidal endothelial cell apoptosis upon reperfusion of the cold ischemic rat liver. Gastroenterology 2000;118:183-91. [Crossref] [PubMed]
- Gujral JS, Bucci TJ, Farhood A, et al. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis? Hepatology 2001;33:397-405. [Crossref] [PubMed]
- Kohli V, Selzner M, Madden JF, et al. Endothelial cell and hepatocyte deaths occur by apoptosis after ischemia-reperfusion injury in the rat liver. Transplantation 1999;67:1099-105. [Crossref] [PubMed]
- Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol 1991;260:G355-62. [PubMed]
- Rüdiger HA, Clavien PA. Tumor necrosis factor alpha, but not Fas, mediates hepatocellular apoptosis in the murine ischemic liver. Gastroenterology 2002;122:202-10. [Crossref] [PubMed]
- Lindert KA, Caldwell-Kenkel JC, Nukina S, et al. Activation of Kupffer cells on reperfusion following hypoxia: particle phagocytosis in a low-flow, reflow model. Am J Physiol 1992;262:G345-50. [PubMed]
- Jaeschke H, Bautista AP, Spolarics Z, et al. Superoxide generation by Kupffer cells and priming of neutrophils during reperfusion after hepatic ischemia. Free Radic Res Commun 1991;15:277-84. [Crossref] [PubMed]
- Selzner N, Rudiger H, Graf R, et al. Protective strategies against ischemic injury of the liver. Gastroenterology 2003;125:917-36. [Crossref] [PubMed]
- Onishi I, Shimizu K, Tani T, et al. JNK activation and apoptosis during ischemia-reperfusion. Transplant Proc 1999;31:1077-9. [Crossref] [PubMed]
- Crenesse D, Gugenheim J, Hornoy J, et al. Protein kinase activation by warm and cold hypoxia- reoxygenation in primary-cultured rat hepatocytes-JNK(1)/SAPK(1) involvement in apoptosis. Hepatology 2000;32:1029-36. [Crossref] [PubMed]
- Lemasters JJ, DiGuiseppi J, Nieminen AL, et al. Blebbing, free Ca2+ and mitochondrial membrane potential preceding cell death in hepatocytes. Nature 1987;325:78-81. [Crossref] [PubMed]
- Trump BF, Goldblatt PJ, Stowell RE. Studies of necrosis in vitro of mouse hepatic parenchymal cells. Ultrastructural alterations in endoplasmic reticulum, Golgi apparatus, plasma membrane, and lipid droplets. Lab Invest 1965;14:2000-28. [PubMed]
- Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology 2003;125:1246-57. [Crossref] [PubMed]
- Mortimore GE, Schworer CM. Induction of autophagy by amino-acid deprivation in perfused rat liver. Nature 1977;270:174-6. [Crossref] [PubMed]
- Pfeifer U. Inhibited autophagic degradation of cytoplasm during compensatory growth of liver cells after partial hepatectomy. Virchows Arch B Cell Pathol Incl Mol Pathol 1979;30:313-33. [PubMed]
- Schneider PD, Gorschboth CM. Limiting ischemic liver injury by interfering with lysosomal autophagy. J Surg Res 1983;34:550-4. [Crossref] [PubMed]
- Czaja MJ, Ding WX, Donohue TM Jr, et al. Functions of autophagy in normal and diseased liver. Autophagy 2013;9:1131-58. [Crossref] [PubMed]
- Shen M, Lu J, Dai W, et al. Ethyl pyruvate ameliorates hepatic ischemia-reperfusion injury by inhibiting intrinsic pathway of apoptosis and autophagy. Mediators Inflamm 2013;2013:461536 [PubMed]
- Rautou PE, Mansouri A, Lebrec D, et al. Autophagy in liver diseases. J Hepatol 2010;53:1123-34. [Crossref] [PubMed]
- Liu T, Zhang Q, Mo W, et al. The protective effects of shikonin on hepatic ischemia/reperfusion injury are mediated by the activation of the PI3K/Akt pathway. Sci Rep 2017;7:44785. [Crossref] [PubMed]
- Wang D, Ma Y, Li Z, et al. The role of AKT1 and autophagy in the protective effect of hydrogen sulphide against hepatic ischemia/reperfusion injury in mice. Autophagy 2012;8:954-62. [Crossref] [PubMed]
- Fiscella M, Zhang H, Fan S, et al. Wip1, a novel human protein phosphatase that is induced in response to ionizing radiation in a p53-dependent manner. Proc Natl Acad Sci U S A 1997;94:6048-53. [Crossref] [PubMed]
- Douarre C, Mergui X, Sidibe A, et al. DNA damage signaling induced by the G-quadruplex ligand 12459 is modulated by PPM1D/WIP1 phosphatase. Nucleic Acids Res 2013;41:3588-99. [Crossref] [PubMed]
- Takekawa M, Adachi M, Nakahata A, et al. p53-inducible wip1 phosphatase mediates a negative feedback regulation of p38 MAPK-p53 signaling in response to UV radiation. EMBO J 2000;19:6517-26. [Crossref] [PubMed]
- Park JY, Song JY, Kim HM, et al. p53-Independent expression of wild-type p53-induced phosphatase 1 (Wip1) in methylmethane sulfonate-treated cancer cell lines and human tumors. Int J Biochem Cell Biol 2012;44:896-904. [Crossref] [PubMed]
- Rossi M, Demidov ON, Anderson CW, et al. Induction of PPM1D following DNA-damaging treatments through a conserved p53 response element coincides with a shift in the use of transcription initiation sites. Nucleic Acids Res 2008;36:7168-80. [Crossref] [PubMed]
- Lu X, Ma O, Nguyen TA, et al. The Wip1 Phosphatase acts as a gatekeeper in the p53-Mdm2 autoregulatory loop. Cancer Cell 2007;12:342-54. [Crossref] [PubMed]
- Batchelor E, Mock CS, Bhan I, et al. Recurrent initiation: a mechanism for triggering p53 pulses in response to DNA damage. Mol Cell 2008;30:277-89. [Crossref] [PubMed]
- Lu X, Nannenga B, Donehower LA. PPM1D dephosphorylates Chk1 and p53 and abrogates cell cycle checkpoints. Genes Dev 2005;19:1162-74. [Crossref] [PubMed]
- Fujimoto H, Onishi N, Kato N, et al. Regulation of the antioncogenic Chk2 kinase by the oncogenic Wip1 phosphatase. Cell Death Differ 2006;13:1170-80. [Crossref] [PubMed]
- Lu X, Bocangel D, Nannenga B, et al. The p53-induced oncogenic phosphatase PPM1D interacts with uracil DNA glycosylase and suppresses base excision repair. Mol Cell 2004;15:621-34. [Crossref] [PubMed]
- Lu X, Nguyen TA, Donehower LA. Reversal of the ATM/ATR-mediated DNA damage response by the oncogenic phosphatase PPM1D. Cell Cycle 2005;4:1060-4. [Crossref] [PubMed]
- Macůrek L, Lindqvist A, Voets O, et al. Wip1 phosphatase is associated with chromatin and dephosphorylates gammaH2AX to promote checkpoint inhibition. Oncogene 2010;29:2281-91. [Crossref] [PubMed]
- Moon SH, Lin L, Zhang X, et al. Wild-type p53-induced phosphatase 1 dephosphorylates histone variant gamma-H2AX and suppresses DNA double strand break repair. J Biol Chem 2010;285:12935-47. [Crossref] [PubMed]
- Moon SH, Nguyen TA, Darlington Y, et al. Dephosphorylation of gamma-H2AX by WIP1: an important homeostatic regulatory event in DNA repair and cell cycle control. Cell Cycle 2010;9:2092-6. [Crossref] [PubMed]
- Song JY, Ryu SH, Cho YM, et al. Wip1 suppresses apoptotic cell death through direct dephosphorylation of BAX in response to gamma-radiation. Cell Death Dis 2013;4:e744 [Crossref] [PubMed]
- Chew J, Biswas S, Shreeram S, et al. WIP1 phosphatase is a negative regulator of NF-kappaB signalling. Nat Cell Biol 2009;11:659-66. [Crossref] [PubMed]
- Li ZT, Zhang L, Gao XZ, et al. Expression and significance of the Wip1 proto-oncogene in colorectal cancer. Asian Pac J Cancer Prev 2013;14:1975-9. [Crossref] [PubMed]
- Bulavin DV, Demidov ON, Saito S, et al. Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat Genet 2002;31:210-5. [Crossref] [PubMed]
- Rauta J, Alarmo EL, Kauraniemi P, et al. The serine-threonine protein phosphatase PPM1D is frequently activated through amplification in aggressive primary breast tumours. Breast Cancer Res Treat 2006;95:257-63. [Crossref] [PubMed]
- Saito-Ohara F, Imoto I, Inoue J, et al. PPM1D is a potential target for 17q gain in neuroblastoma. Cancer Res 2003;63:1876-83. [PubMed]
- Le Guezennec X, Brichkina A, Huang YF, et al. Wip1-dependent regulation of autophagy, obesity, and atherosclerosis. Cell Metab 2012;16:68-80. [Crossref] [PubMed]
- Brichkina A, Bulavin DV. WIP-ing out atherosclerosis with autophagy. Autophagy 2012;8:1545-7. [Crossref] [PubMed]
- Choi J, Nannenga B, Demidov ON, et al. Mice deficient for the wild-type p53-induced phosphatase gene (Wip1) exhibit defects in reproductive organs, immune function, and cell cycle control. Mol Cell Biol 2002;22:1094-105. [Crossref] [PubMed]
- Uyanik B, Grigorash BB, Goloudina AR, et al. DNA damage-induced phosphatase Wip1 in regulation of hematopoiesis, immune system and inflammation. Cell Death Discov 2017;3:17018. [Crossref] [PubMed]
- Ramadori P, Kroy D, Streetz KL. Immunoregulation by lipids during the development of non-alcoholic steatohepatitis. HepatoBiliary Surg Nutr 2015;4:11-23. [PubMed]
- Maddika S, Ande SR, Panigrahi S, et al. Cell survival, cell death and cell cycle pathways are interconnected: implications for cancer therapy. Drug Resist Updat 2007;10:13-29. [Crossref] [PubMed]
- Wu MP, Zhang YS, Zhou QM, et al. Higenamine protects ischemia/reperfusion induced cardiac injury and myocyte apoptosis through activation of beta2-AR/PI3K/AKT signaling pathway. Pharmacol Res 2016;104:115-23. [Crossref] [PubMed]
- Yu H, Zhang H, Zhao W, et al. Gypenoside Protects against Myocardial Ischemia-Reperfusion Injury by Inhibiting Cardiomyocytes Apoptosis via Inhibition of CHOP Pathway and Activation of PI3K/Akt Pathway In Vivo and In Vitro. Cell Physiol Biochem 2016;39:123-36. [Crossref] [PubMed]
- Roy S, Benz F, Luedde T, et al. The role of miRNAs in the regulation of inflammatory processes during hepatofibrogenesis. HepatoBiliary Surg Nutr 2015;4:24-33. [PubMed]
- Ghoshal S, Fuchs BC, Tanabe KK. STAT3 is a key transcriptional regulator of cancer stem cell marker CD133 in HCC. Hepatobiliary Surg Nutr 2016;5:201-3. [Crossref] [PubMed]