
Characteristics and treatment of patients with neuroendocrine carcinoma of the gastroesophageal junction: an analysis of 13 cases
Introduction
Neuroendocrine neoplasms (NENs) are prevalent neuroendocrine differentiated epithelial tumors, originating from dispersed cells in the endocrine system, portrayed by their peptide producing ability, triggering a range of hormone related syndromes (1). Numerous scholars narrated gastroenteropancreatic NENs (GEP-NENs) related management ranging from: epidemiology of, to diagnosis and pathology in Caucasian patients (American, European) (2-4). Asian NEN cases are infrequent with scarce data reported regarding Asian patients especially in China. The Surveillance, Epidemiology and End Results (SEER) database in the US concurs 61% of NENs to be GEP-NENs, ranging in predominance from the rectum (17.7%), small intestine (17.3%), colon (10.1%), pancreas (7.0%), stomach (6.0%), and appendix (3.1%) (2). Internationally, gastroesophageal junction (GEJ) NENs are infrequently reported. In China, a 10-year multi-center (Level 3 hospitals) retrospective study from 2001–2010 assembled patient information diagnosed with GEP-NENs, sharing common occurrence in Chinese patients to be in the pancreas (31.5%) and rectum (29.6%). Neither of these studies reported NENs of GEJ (5). WHO distributed an updated classification of NENs in 2010, revising NENs definition to include all tumors involving neuroendocrine differentiation (6). Digestive neuroendocrine carcinoma (NEC) as well as mixed adeno-NEC (MANEC) is extremely rare and highly malignant neoplasm which is classified as classes 3 and 4. NEC/MANEC are a very aggressive type of cancer, especially making it a diagnostic challenge for clinicians when the tumor located in GEJ (7). This present study rationale was to implement an in-hospital (PUMCH) analysis of GEJ-NEC/MANEC. With intention to share new evidence regarding progression and management of such rare tumors.
Methods
Patients
Positively diagnoses GEJ-NEC/MANEC patients were retrospectively observed in accordance to the 2000 WHO classification from January 2006 to January 2017 at the Department of Thoracic Surgery, PUMCH. All included patients were pathologically confirmed primary GEJ-NEC/MANEC, with available original pathology reports.
Data collection
At the PUMCH, all patients’ original medical records and files are well preserved in portfolios ever since 1920s. With the help of the electronic medical record system (EMR), analysis of collected parameters include: clinical features (age, gender, symptoms, and physical signs), diagnostic (radio-imagery and pathology), tumor descriptions (primary location, size, stage and grading in accordance to WHO 2010 classification), medical intervention (surgery, adjuvant therapy), etc. Cancer staging followed routine tumor node metastasis [TNM 7th edition, Union for International Cancer Control (UICC)] methodology in relation to the anatomical loci. Grading was determined through morphology and increased activity.
Results
Patients’ clinical characteristics
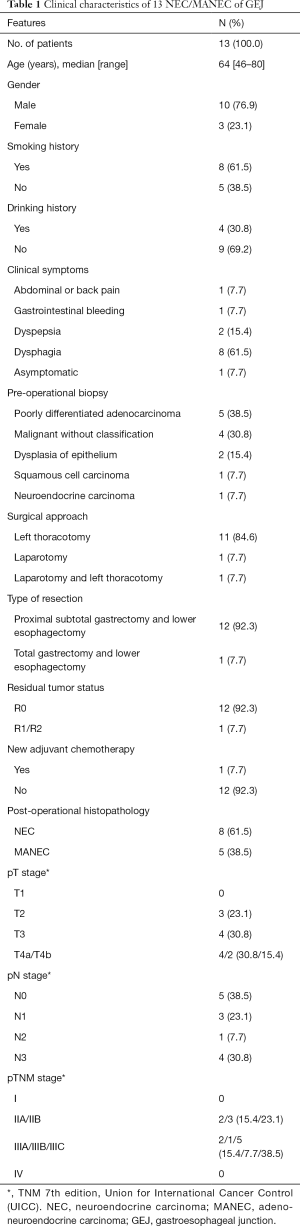
Thirteen patients were included in the present study. Ten (76.9%) patients were male, 3 (23.1%) female, with 3.3:1 male-to-female ratio. Mean age 64.0±9.9 years. The most frequent initial symptoms and signs were typical dysphagia (n=8, 61.5%), followed by dyspepsia (n=2, 15.4%), and other non-specific symptoms (Table 1). One case (7.7%) was asymptomatic and incidental finding during routine health examinations. Eight patients (61.5%) had of history of smoking and 4 patients (30.8%) admitted regular drinking.
Full table
Treatment
All patients received gastroscopy biopsy in PUMCH or other hospitals before the surgery. NEC was considered only in 1 case (7.7%). Majority of biopsy results were poorly differentiated adenocarcinoma 5 cases (38.5%) and malignant without classification 4 cases (30.8%). Only 1 patient (7.7%) received neo-adjuvant chemotherapy with cisplatin and paclitaxel to shrink primary tumor. Each patient received optimal surgical regimen and treatment, 11 cases were treated through left thoracotomy (84.6%), 1 case (7.7%) with laparotomy and 1 case (7.7%) combined approaches. Twelve patients (92.3%) underwent proximal subtotal gastrectomy and lower esophagectomy. Another 1 patient received total gastrectomy and lower esophagectomy. R0 resection rate was 92.3%.
Tumors’ characteristics
Overall, post operational histopathology revealed NEC in 8 cases (61.5%) and MANEC in 5 cases (38.5%). The maximum diameter of tumor ranged from 2 to 8 cm (median 4.3 cm). For pTNM stage, 2 cases (15.4%) were stage IIA, 3 (23.1%) were stage IIB, 8 (61.5%) were stage 3. The mitotic count was higher than 20/10 HPFs in 4 cases (30.8%). The Ki-67 index was >75% in 8 cases (61.5%). Synaptophysin (Syn) was positive in all cases and chromogranin A (CgA) staining positive rate was 76.9%. Lymph node metastasis was noted in 8 of 13 cases (61.5%).
Discussion
The etiology and pathogenesis of NEC/MANEC is poorly understood and controversial (8). It is even challenging to be diagnosed when the tumor located in GEJ. For the very rare case was reported NEC/MANEC of GEJ worldwide, our retrospective study to investigated clinical traits, pathological classification and treatment of this extremely rare disease.
An increased incidence of NENs during the last 10 years is observed, attributing to improved clinical and pathological experience in identification of his disease, and increased clinical awareness and endoscopic scrutiny (3). Early data from the European countries proposed gastrointestinal NENs occurrence to be 0.0025% per year. While recent SEER program published a fluctuation from 0.0019% [1973] to 0.00525% [2004], with 2.65 times as many GEP-NEN diagnosis in 2007 compared to 1973 (3). NEC/MANEC is even rarer and high-quality epidemiological data is lacking.
In our experience, same with other esophageal neoplasms, patients seek medical help mostly because of progressive dysphagia. Aiming for primary tumor diagnosis, a multi-model tactic is recommended. Biochemistry, upper gastroenterography, CT, and MRI are recommended. Besides, other imaging modalities would help may include endoscopy, endoscopic ultrasound (EUS), digital subtraction angiography (DSA) (9). Histopathology is required to confirm the diagnosis, but NEC/MANEC is difficult to diagnosis by endoscopy biopsy due to our experience. Some cases were diagnosed as “malignant without classification” or “poorly differentiated adenocarcinoma” because of the histological difficulty in differentiating, or because there was not enough information, or for the other reasons. As recommended, multidisciplinary teams (MDTs) at medical centers should guide absolute management of all NENs variety patients. MDT make up includes NENs specialists from various departments such as oncology, endocrinology, gastroenterology, surgery, radiology, histopathology and nursing. Pathology is still the diagnostic gold standard, pathology review and diagnosis are limited to the MDT pathologist.
According to whether have hormone-related clinical manifestations, GEP-NENs is classified into two categories: non-functioning and functioning tumors. In all NENs, presentation may be non-specific symptoms for example abdominal or back discomfort, dysphagia, nausea and vomiting, intestinal bleeding, etc. Functioning tumors cause symptoms dependent on the peptide hormone released. Study results testify NENs patients to be under the immediate threat of syndrome related features rather than malignancy itself. Of 13 cases in this study are all non-functioning, presenting with only primary tumor mass effect.
Pathological characterization and classification of NENs right now is still based on the WHO 2010 classification, the UICC TNM (7th edition), and the European Neuroendocrine Tumour Society (ENETS) site-specific T-staging system. While in the 8th edition of the American Joint Committee on Cancer (AJCC) published in 2017, staging of epithelial cancers of the esophagus and EGJ presents separate classifications for clinical (cTNM), pathologic (pTNM), and post neoadjuvant (ypTNM) stage groups. The EGJ was also redefined in the 8th edition: adenocarcinomas with epicenters no more than 2 cm into the gastric cardia are staged as esophageal adenocarcinomas, and those extending further are staged as stomach cancers, as known as 2 cm principle.
In WHO 2010 classification, NEC are defined as poorly differentiated tumors, with small or large cells, conveying neuroendocrine markers CgA and Syn, and with a high proliferative index (grade 3 with a Ki-67 >20%). While MANEC is defined as a tumor with two components: adenocarcinoma as well as neuroendocrine tumor, mostly considered a NEC (10). Based on the WHO criteria, each component makes up at least 30% of the tumor. Both NEC and MANEC are rare types of tumor, with extremely rare EGJ occurrence, hence challenging diagnosis.
Despite the controversy surrounding the treatment of NENs, treatment objective is curative if possible, with prolonged disease control and symptom free maintenance of patients, ensuring quality of life (QoL). Consensus states surgery to be the only curative treatment for NENs, and should be prioritized as treatment in resectable cases (11). Challenges specific to NENs involve the definition of local/ distant tumor extent, the extent of local and distant tumors, detection of synchronous non-NENs, discovery of other complications and nutrition status (12). While the treatment plan should be modified accordingly, within a MDT framework. With NENs, research shows low metastasis with primary lesions <2 cm in diameter. Prior to treatment design, the following confirmations are required: tumor location and coverage, histologic grading, metastases, and secretory profile. For non-surgery candidates (patient unfit), comprehensive aim is symptom control and QoL maintenance. According to the reports, comprehensive treatment choices for non-resectable NEC/MANEC include chemotherapy, biotherapy, locoregional treatments including ablation, targeted radionuclide therapy. Recent advanced studies share great promise in NENs treatment with biological and targeted regimen (13). Patients with strong somatostatin receptor-positive tumors may consider experimental treatment using peptide radionuclide receptors, or consider this as second- or third-line therapeutic considerations. And if possible, patients with NEC/MANCE should enter formal new drug treatment trials.
NENs are slow-growing tumors, survival depends on several factors. SEER and NRC studies report the highest 5-year survival rates to be rectal primary tumors (74–88%), lowest were pancreatic primaries (27–43%) (2). And SEER data reports insignificant change between 1973–2002 regarding 5-year survival of all NENs patients regardless of primary site and degree of metastasis, remaining at 60–65% (2-4). Survival length of survival is directly correlated to both disease extent upon diagnosis and tumor differentiation. NEC/MANEC tumors have lower 5-year survival rates: local metastasis <38%, general spread 21%, distant metastasis <4%. Research shows tumor Ki-67 proliferation index, histopathology, size and location, patient age, all influence survival (2).
Conclusions
In sum, NEC/MANEC of GEJ are a kind of extremely rare tumors. It is difficult to diagnosis before operation and surgery is still the primary treatment for resectable GEJ-NEC/MANEC.
Acknowledgments
We acknowledge the challenging work of multidisciplinary teams (MDTs) in PUMCH, including but not limited to thoracic surgeons, gastroenterologists, pathologists, endocrinologists, radiologists, etc.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.12.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). According to the hospital’s ethical review board, ethical review is not necessary for such retrospective research. Written informed consents were obtained from the patients for the publication of this report and any accompanying files and images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- La Rosa S, Inzani F, Vanoli A, et al. Histologic characterization and improved prognostic evaluation of 209 gastric neuroendocrine neoplasms. Hum Pathol 2011;42:1373-84. [Crossref] [PubMed]
- Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [Crossref] [PubMed]
- Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am 2011;40:1-18. vii. [Crossref] [PubMed]
- Hemminki K, Li X. Incidence trends and risk factors of carcinoid tumors: a nationwide epidemiologic study from Sweden. Cancer 2001;92:2204-10. [Crossref] [PubMed]
- Fan JH, Zhang YQ, Shi SS, et al. A nation-wide retrospective epidemiological study of gastroenteropancreatic neuroendocrine neoplasms in china. Oncotarget 2017;8:71699-708. [PubMed]
- Turaga KK, Kvols LK. Recent progress in the understanding, diagnosis, and treatment of gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin 2011;61:113-32. [Crossref] [PubMed]
- Ilett EE, Langer SW, Olsen IH, et al. Neuroendocrine Carcinomas of the Gastroenteropancreatic System: A Comprehensive Review. Diagnostics (Basel) 2015;5:119-76. [Crossref] [PubMed]
- Veits L, Lang-Schwarz C, Volkholz H, et al. Mixed adenoneuroendocrine carcinoma (MANEC) of the esophagogastric junction predominantly consisting of poorly differentiated neuroendocrine carcinoma. Endoscopy 2013;45 Suppl 2 UCTN:E16-7.
- Xu EX. Professor Donald N. Reed: the development of endoscopy. Hepatobiliary Surg Nutr 2016;5:193-4. [PubMed]
- La Rosa S, Marando A, Sessa F, et al. Mixed adenoneuroendocrine carcinomas (MANECs) of the gastrointestinal tract: an update. Cancers (Basel) 2012;4:11-30. [Crossref] [PubMed]
- Delle Fave G, Kwekkeboom DJ, Van Cutsem E, et al. ENETS consensus guidelines for the management of patients with gastroduodenal neoplasms. Neuroendocrinology 2012;95:74-87. [Crossref] [PubMed]
- Liu Y, Xue X. Systematic review of peri-operative nutritional support for patients undergoing hepatobiliary surgery. Hepatobiliary Surg Nutr 2015;4:304-12. [PubMed]
- Martin RC 2nd. Use of irreversible electroporation in unresectable pancreatic cancer. Hepatobiliary Surg Nutr 2015;4:211-5. [PubMed]


