
Clinical efficacy of intravenous chemotherapy alone versus intravenous combined with intraperitoneal chemotherapy for newly diagnosed gastric cancer with malignant ascites
Introduction
Gastric cancer (GC) is one of the most common malignant tumours in the world. According to the “Global Cancer statistics, 2012” data released by the authoritative journal “CA: A Cancer Journal for Clinicians”, there have been more than 950,000 new cases of GC in the world, ranking 5th overall; in addition, global cases of GC deaths number approximately 730,000, ranking 3rd (1). The “Cancer Statistics in China, 2015”, published on-line by CA in 2016, showed that the morbidity and mortality of GC both ranked at the top of malignant tumours in China (2). Considerable numbers of Chinese GC patients are already at the advanced stage when they are first diagnosed. Malignant ascites is one of the common symptoms or signs of GC at the advanced stage and is an important factor influencing the quality of life (QOL) and prognosis of GC patients. This condition has become an urgent issue to be solved in clinical settings; however, currently, there is still no standard treatment regimen for malignant ascites (3). Therefore, in-depth searches for effective measures for controlling malignant ascites to relieve the pain of these GC patients and to improve their prognosis have important significance (4). This study performed retrospective analyses on the conditions of diagnosis and treatment of 33 cases of newly diagnosed GC patients combined with malignant ascites admitted in the Fujian Medical University Union Hospital, China, between September 2010 and September 2015, who had complete clinical data. The results of those analyses are reported here.
Methods
General data
Thirty-three cases of newly diagnosed GC patients combined with malignant ascites who were diagnosed, treated, and hospitalized and had complete clinical data in the Fujian Medical University Union Hospital between September 2010 and September 2015 were enrolled. There were 16 men and 17 women. The ages were between 21.00–73.00 years, the median age was 48.00 years, and the mean age was 47.61±2.60 years. Patients were pathologically confirmed to have primary GC by gastroscopy and to have small to large amounts of ascites confirmed by computed tomography/magnetic resonance imaging (CT/MRI) or combined with ultrasound before diagnosis and treatment. Malignant ascites was diagnosed by the combination of ascites biochemistry, abdominal imaging, and peritoneal exfoliative cytology examinations. The pathological types of all cases were classified as follow: 20 cases of adenocarcinoma, 11 cases of signet ring cell carcinoma, 1 case of mucinous carcinoma, and 1 case of squamous cell carcinoma. Among patients with metastasis, 2 cases only had malignant ascites; 12 patients only had one location of metastasis besides the malignant ascites, including 6 cases in the peritoneum, 3 cases in ovaries, 2 cases in retroperitoneal lymph nodes, and 1 case in the abdominal cavity. Thirteen patients had two locations of metastasis besides the malignant ascites, including 3 cases in the peritoneum and retroperitoneal lymph nodes, 2 cases in the abdominal cavity and peritoneum, 2 cases in the abdominal cavity and the liver, 1 case in the liver and the uterus, 1 case in the liver and the perigastric lymph nodes, 1 case in the peritoneum and the perigastric lymph nodes, 1 case in the abdominal cavity and an ovary, 1 case in the lung and the transverse colon, and 1 case in the hilar lymph nodes and the retroperitoneal lymph nodes. Six patients had more than two locations of metastasis besides the malignant ascites, including 4 cases with metastasis in three locations and 2 cases with metastasis in four locations. The amount of ascites in the two groups was listed as follow: with 12 cases of small amount of ascites and 6 cases of middle-large amount of ascites in the simple group, as well as 7 cases of small amount of ascites and 9 cases of middle-large amount of ascites in the combined group, the difference between the two groups was not statistically significant (χ2=1.340, P=0.247). The physical condition scores of patients before treatment used the Eastern Cooperative Oncology Group (ECOG) score as the standard; the average scores were all ≤3, indicating that there were no severe heart, liver, and kidney dysfunctions and that the expected survival was ≥3 months. The diagnosis and treatment processes of the above patients were retrospectively analysed. Patients were divided into two groups based on the treatment methods they received. Patients who only received intravenous chemotherapy alone were defined as the simple group; there were 18 cases, including 10 men and 8 women, the ages were 22.00–73.00 years, the median age was 51.50 years, and the mean age was 51.72±3.52 years. Patients who received intravenous combined with intraperitoneal chemotherapy (IPC) were defined as the combination group; there were 15 cases, including 6 men and 9 women, the ages were 21.00–66.00 years, the median age was 45.00 years, and the mean age was 42.67±3.58 years. The mean ages of patients between the simple group and the combination group were not significantly different (P>0.05). Gender, age composition, ECOG before treatment, clinical stages, and the pathological data of advanced GC patients of these two groups were equally distributed and all had comparability (Table 1). Ethical approval for the study was provide by the Ethics Committee of Fujian Medical University Union Hospital (Ref. No. 2017KY082).

Full table
Treatment methods
Chemotherapy regimens
Intravenous chemotherapy regimens
Regimens were chosen based on the Clinical Practice Guidelines for Gastric Cancer (Chinese version) of the National Comprehensive Cancer Network (NCCN) and the “Standard for diagnosis and treatment of gastric cancer (2011 edition)” released by the Medical Administration of the Chinese Ministry of Health (5). Drugs included taxanes (paclitaxel and docetaxel), fluorouracil (5-fluorouracil, tegafur, and capecitabine), and platinum (cisplatin and oxaliplatin). The above-mentioned drugs were used as a single drug or as a combination of two drugs, and 1 cycle was 2 or 3 weeks.
Intravenous combined with IPC regimens
The intravenous chemotherapy regimens were the same as those described above. The drugs used in the IPC regimens included 5-fluorouracil, tegafur, cisplatin, oxaliplatin, and mitomycin used as a single drug or as a combination of two drugs. During each cycle of the intravenous chemotherapy, the IPC could be performed 1–3 times.
IPC methods
A traditional intraperitoneal perfusion chemotherapy method was performed without using an intraperitoneal hyperthermic perfusion chemotherapy machine. Patients were placed in a supine position, and either the anti-McBurney point was used as the puncture point, or ultrasonic exploration positioning guidance was used. Conventional disinfection and draping were performed, and 2% lidocaine was used for anaesthesia. Next, a deep vein puncture needle entered through the local anaesthesia location perpendicularly to the skin surface. When the needle was confirmed to have entered the correct location, a guide wire was placed. The puncture needle was removed, and an 8F deep venous catheter was placed into the abdominal cavity along the guide wire. After it was confirmed that patients were not experiencing obvious discomfort, 1,000 mL of normal saline was dropped rapidly, followed by perfusion of 500 mL of chemotherapeutic drugs and normal saline. After the operation, the puncture location was wrapped with dressing. Patients were told to change their body positions frequently within 2–4 hours to facilitate the even distribution of chemotherapeutic drugs in all locations of the abdominal cavity.
Efficacy evaluation
Patients received routine blood, liver and kidney function, coagulation function, and tumour marker examinations, chest CT, and all abdominal CT/MRI or CT combined with ultrasound before the first treatment. Patients in the simple group received corresponding follow-up examinations after receiving 2–3 cycles of intravenous chemotherapy, and patients in the combination group received corresponding follow-up examinations after receiving 2–3 cycles of intravenous therapy and IPC during this period to evaluate the short-term objective efficacy and adverse reactions. Furthermore, the median survival period and the survival rate in the corresponding time period were obtained through follow-up with patients to evaluate the long-term efficacy.
Evaluation of the short-term objective efficacy
The objective efficacy of advanced GC was evaluated according to Response Evaluation Criterion Solid Tumors (RESCIST version 1.1) provided by World Health Organization (WHO), including complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). Objective response rates (ORR) was defined by the FDA as the sum of CRs plus PRs and disease control rates (DCR) as the sum of CRs, PRs plus SDs.
Evaluation of ascites efficacy
There are four categories for evaluating the efficacy of ascites according to the criteria established by WHO as follow: (I) CR: ascites disappeared completely for more than 1 month; (II) PR: malignant ascites was reduced by at least 50% and the state lasts more than 1 month; (III) SD: less than 50% of ascites was reduced; (IV) PD: ascites relapsed into the condition as pretreatment or progressed. The ORRs and DCRs of ascites were defined as the sum of CRs plus PRs and the sum of CRs, PRs plus SDs, respectively.
QOL assessment
Using ECOG scores as the assessment to analysis every patient’s change of the QOL after treatment.
Survival conditions
Median survival time (MST) refers to the survival time when the cumulative survival rate is 0.5. The MST and 12-, 18-, 24-, 30-, 36-, 60-month survival rates were compared between two groups.
Determination of adverse reactions
Adverse reactions were observed and recorded according to America National Cancer Institute-Common Terminology Criteria for Adverse Events 3.0 (NCI-CTC 3.0). Grade I: mild adverse reactions; grade II: moderate adverse reactions; grade III: severe adverse reactions; grade IV: life-threatening or disabling adverse reactions; grade V: death related to adverse reactions. The incidence rate of adverse events (AE) was calculated by numbers of grades III–IV AE/total number of patients in each group.
Statistical analysis
Statistical analyses were performed using SPSS software 22.0 (SPSS Inc., Chicago, IL, USA). Continuous variable results were presented as mean ± standard deviation (SD) or median with range. Associations among various categorical variables were analyzed by Pearson χ2 test or Fisher exact test and noncategorical variables were evaluated by t-tests. Subsequently, survival curves were estimated using the Kaplan-Meier method and compared by the Breslow test. Statistical significance was set at P<0.05.
Follow-up
All patients, after accepting treatment, were followed up by telephone interviews and outpatient visits as well several times. The survival time was defined as the time from the date of first chemotherapy to the last contact time, September 2016, or the date of death. All patients were followed up with a follow-up rate of 100%.
Results
Evaluation of short-term objective efficacy
The objective response rates of advanced GC patients in the simple and combination groups were 11.11% and 40.00%, respectively (P>0.05). The comparison results indicated that the difference was not statistically significant. The DCRs of advanced GC patients in the simple and combination groups were 44.44% and 80.00%, respectively (P<0.05). The comparison results indicated that the differences were statistically significant (Table 2).

Full table
Evaluation of local efficacy on malignant ascites
The local ORRs of malignant ascites of advanced GC patients in the simple and combination groups after treatment were 22.22% and 66.67%, respectively; the χ2 test result showed that P<0.05; the comparison results indicated that the difference was statistically significant. The local DCRs of malignant ascites of advanced GC patients in the simple and combination groups after treatment were 55.56% and 100.00%, respectively (χ2 test, P<0.05); the comparison results indicated that the difference was also statistically significant (Table 3).

Full table
Analysis of survival conditions
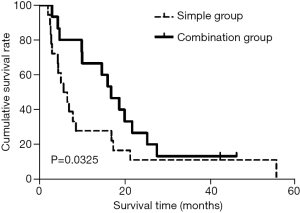
The MSTs of advanced GC patients in the simple and combination groups were 6.03±1.59 and 17.03±2.62 months, respectively; the comparison results indicated that the difference was statistically significant (χ2=4.41, P<0.05). The 12-, 18-, 24-, 30-, 36-, and 60-month survival rates of the simple group were 27.8% (5/18), 16.7% (3/18), 11.1% (2/18), 11.11% (2/18), 11.11% (2/18), and 5.6% (1/18), respectively, which were significantly lower than the survival rates of 66.7% (10/15), 46.7% (7/15), 26.7% (4/15), 13.3% (2/15), 13.3% (2/15), and 13.3% (2/15) for patients in the combination group; the comparison results indicated that there were significant differences (χ2=5.55, 5.09, 4.75, 4.41, 4.41, 4.41, all P<0.05) (Table 4). The results of survival analyses using the Kaplan-Meier methods showed that the differences in the survival periods of advanced GC patients between these two groups were also statistically significant (Figure 1).
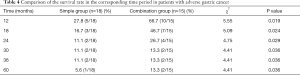
Full table
Comparison of quality of life
The ECOG scoring results showed that the mean ECOG scores of advanced GC patients in the simple group before and after treatment were 1.28±0.18 and 1.11±0.20, respectively; the comparison results indicated that there was no statistical significance (Z=−0.958, P>0.05). The mean ECOG score in the combination treatment group after treatment (1.13±0.17) was significantly lower than that before treatment (1.67±0.16); the comparison results indicated that there was statistical significance (Z=−2.480, P<0.05) (Table 5).

Full table
Determination of adverse reactions
The adverse reactions of advanced GC patients in these two groups after treatment were mainly degrees 0–II nausea and vomiting, diarrhoea, and hand-foot syndrome, etc. The incidence rate of AE in the simple group was 33.33% (6/18), which was lower than the incidence of 53.33% (8/15) in the combination group; however, the difference between these two groups was not statistically significant (χ2=1.34, P>0.05). The degrees III–IV adverse reactions were mainly the presentations of leukopenia, thrombopenia, gastrointestinal reaction, and bone marrow suppression. They were all within the controllable range and were relieved within a short time after symptomatic treatment, and there were no treatment-related deaths (Table 6).

Full table
Discussion
GC is currently among the cancers with the highest incidence in the world. There are almost 1 million new cases every year, and patients in China account for approximately 42% of all new cases. In addition, GC is also the second cause of cancer death in the world; its mortality in Asian countries such as Japan, South Korea, and China ranks 1st among malignant tumours (1). Currently, compared with developed countries such as Japan and South Korea, China still has not generally implemented relevant GC screening studies. Therefore, the oncology of GC develops into the 3-high and 3-low characteristics, including high incidence, high metastasis, high mortality, low early diagnosis rate, low resection rate, and low 5-year survival rate. The percentage of GC patients who are at the early stage when they seek clinical treatment is lower than 10%; the majority of patients are already at the local progression stage or the advanced stage, and of these, a certain number of patients already have GC combined with ascites when they are first diagnosed. The formation of ascites in GC is usually the result of the functions of many factors at different stages, including peritoneal metastasis of tumours, obstruction of lymphatic return, malnutrition, and concurrent infection and perforation. Symptoms such as abdominal distension, abdominal pain, anorexia, and dyspnoea resulting from malignant ascites are usually difficult to eradicate, significantly reduce the QOL of patients, and are closely associated with disease aggravation and prognosis (6,7). Therefore, effective control of malignant ascites has very important significance in the comprehensive treatment of advanced GC.
In the past, scholars have mainly used abdominal paracentesis drainage to treat malignant ascites; however, simple abdominal paracentesis drainage can only be used for palliative relief of the symptoms of ascites compression and cannot inhibit the growth of metastatic tumour cells in the peritoneum or decrease ascites production. In addition, repeated drainage will cause protein loss, malnutrition, and cachexia, aggravating patients’ pain, and can even accelerate the progression of disease courses and shorten patients’ survival times. Relevant studies have shown that the presence of malignant ascites in patients with malignant tumours suggested peritoneal implantation and metastasis. The presence of the blood-brain-barrier-like peritoneal-plasma barrier between veins and the peritoneum in the body makes it difficult for intravenous chemotherapeutic drugs to reach the peritoneum; therefore, their efficacy on malignant ascites has been poor (8). In 1978, Dedrick et al. (9) established a pharmacokinetic model of peritoneal drug administration, the “two-chamber model”, composed of the systemic circulation and two chambers of the abdominal cavity. After drug administration, excretion was relatively rapid due to the presence of kidney clearance and liver metabolism in the systemic circulation. Therefore, the concentration of chemotherapeutic drugs in the plasma was significantly lower than that in the abdominal cavity, highlighting the particular advantages of IPC. Subsequent studies also confirmed that IPC could significantly increase the effective concentrations of drugs and prolong their action times. Kuzuya et al. intraperitoneally injected 5-fluorouracil and showed that the area under the concentration-time curve (AUC) in the peritoneal fluid was 1,000 times higher than that of the plasm AUC (10). Fushida et al. (11) performed intraperitoneal administration of paclitaxel and showed that the peak concentration of the drug was 550–2,000 times higher than that in plasma. High concentrations of chemotherapeutic drugs can be more ideally distributed in all locations in the abdominal cavity; in addition, improvement of the peritoneal microcirculation can directly produce cytotoxic effects on free cancer cells (FCCs) in the abdominal cavity and micrometastatic foci in the peritoneum, kill intraperitoneal inflammatory cells and platelets, and reduce the release of growth factors to block their promotion functions on tumour cell proliferation (12,13). Studies have also shown that intraperitoneal chemotherapeutic drugs are absorbed by capillaries to enter the human liver through the portal vein. Increasing the drug concentration in the portal vein to facilitate drug delivery to tumour cells in the portal vein system and micrometastatic foci in the liver parenchyma can control liver metastasis. After being absorbed by the lymphatic tubes, chemotherapeutic drugs enter into the intraperitoneal lymphatic tract to produce lymphatic chemotherapy to rapidly kill FCCs obstructing the lymphatic tract, re-establishing the patency of the lymphatic tract drainage, which can help to control and eliminate ascites. Furthermore, most chemotherapeutic drugs in IPC are metabolized by the liver and enter into the systemic circulation in non-toxic forms, which not only reduces the damage of the immune system by systemic adverse reactions of chemotherapeutic drugs but also increases the tolerance of the body to anti-tumour drugs to achieve the more ideal functions of large doses, high concentrations, and good effects (14,15).
Until now, compared with intravenous chemotherapy, clinical practices have confirmed that IPC has excellent local anti-tumour treatment effects and ascites control rates in GC with malignant ascites. Its combination with intravenous chemotherapy can change the anti-tumour effects on FCCs in malignant ascites and metastatic tumours in the abdominal cavity, effects that simple intravenous chemotherapy cannot achieve. IPC is an excellent comprehensive measure that can address both systemic and local treatment of GC combined with malignant ascites, significantly increase the QOL of patients, delay disease progression, and have active effects in the prolongation of patient survival. In 1996, Wang et al. (16) performed IPC on seven GC patients combined with malignant ascites using 5-fluorouracil combined with cisplatin and reported an ascites disappearance rate of 85.7% and a surgical resection rate of 57.1%. In 2005, Nakamura et al. (17) reported one case of T3N2MOHOP1CY1M0 GC in a patient combined with ascites. After intraperitoneal perfusion of paclitaxel or docetaxel for chemotherapy, the ascites was significantly reduced, and intraperitoneal FCCs disappeared. In 2006, Kobayashi et al. (18) performed intravenous injection of paclitaxel at 60–80 mg/m2 on two advanced GC patients combined with peritoneal metastasis and malignant ascites and showed that the plasma peak value could be reached immediately and could be rapidly reduced to 85 ng/mL after 24 h; however, the drug concentration in the abdominal cavity gradually increased, reaching the plasma, where it was maintained for more than 72 h. After two courses of treatment, the ascites in these two cases disappeared rapidly. In 2007, Kodera et al. (19) performed chemotherapy using intraperitoneal perfusion of paclitaxel at 60 mg/m2 for every week on four GC combined with malignant ascites patients. The regimen of 3 continuous weeks of treatment followed by 1 week of rest for at least 2 continuous cycles was used, and ascites completely disappeared in two cases. In 2008, Shimoyama et al. (20) performed chemotherapy using intraperitoneal perfusion of paclitaxel (70 mg/m2; 3 continuous weeks followed by 1 week of rest) on a 62-year-old GC patient with a large amount of ascites. The ascites disappeared rapidly, the patient’s abdominal pain was significantly relieved, and the patient survived for more than 36 months. In 2010, one phase II clinical trial implemented by Ishigami et al. (21) showed that after intravenous injection of paclitaxel at 50 mg/m2 and intraperitoneal perfusion of paclitaxel at 20 mg/m2 each week on 21 GC combined with malignant ascites patients, the ascites completely disappeared in 13 cases, the MST was 22.5 months, and the 1- and 2-year survival rates were 78% and 46%, respectively. The results of one registered clinical study [UMIN (X) 0002850] reported by Yamaguchi et al. (22) in 2013 on 35 advanced GC patients with peritoneal metastasis, using the preoperative neoadjuvant intraperitoneal-systemic chemotherapy protocol (NIPS) with methotrexate and tegafur for an average of 11 treatment courses, showed that the ascites disappearance or significant reduction rate was 68%, the FCC negative rate reached 97%, and the MST was 17.6 months. In addition, 21 patients received total and subtotal gastrectomy because their cancer lesions were effectively controlled; the 1- and 2-year survival rates of 35 patients reached 77.1% and 44.8%, respectively. In 2014, Kitayama et al. (4) also used the above methotrexate regimen combined with a tegafur NIPS regimen (2–16 treatment courses, with an average of 5 treatment courses) on 64 cases of GC combined with peritoneal metastasis or malignant ascites confirmed by laparoscopic exploration. Thirty-four cases experienced significant reductions or partial disappearances of intraperitoneal metastatic nodules and ascites that decreased or disappeared, and their FCCs became negative; therefore, the patients could receive surgical treatment. Their MST was 25.4 months, and the 1-year survival rate was 82%. Patients who did not receive conversion surgery had an MST of 12.1 months and a 1-year survival rate of 26%. These results suggested that IPC combined with systemic chemotherapy greatly increased the chance of receiving conversion surgery therapy and could prolong and improve the survival times of some GC patients combined with peritoneal metastasis or malignant ascites.
The short-term efficacy evaluation results in this retrospective study showed that the ORR and DCR of advanced GC patients combined with malignant ascites who received simple intravenous chemotherapy were 11.11% and 44.44%, respectively, which were significantly lower than the 40.00% and 80.00% results for patients who received intravenous combined with IPC. The local ORR and DCR of malignant ascites from advanced GC patients in the simple group after treatment were 22.22% and 55.56%, respectively, which were also significantly lower than the 66.67% and 100.00% results of advanced GC patients in the combination group. The long-term efficacy evaluation results showed that the MST of advanced GC patients in the simple group was 6.03±1.59 months, which was shorter than 17.03±2.62 months in the combination group. In addition, the 12-, 18-, 24-, 30-, 36-, and 60-month survival rates of advanced GC patients in the simple group were 27.8%, 16.7%, 11.1%, 11.1%, 11.1%, and 5.6%, which were significantly lower than the survival rates of 66.7%, 46.7%, 26.7%, 13.3%, 13.3%, and 13.3% of the advanced GC patients in the combination group. Comparison of advanced GC patients in these two groups revealed that the differences were also statistically significant (χ2=5.55, 5.09, 4.75, 4.41, 4.41, 4.41, P<0.05). Analyses of the reasons for these differences indicated that the benefit of survival in advanced GC patients in the combination group should come from the control of local malignant ascites by IPC, thus making important contributions to improving the systemic tumour burden in the body. Furthermore, observations of changes in the physical conditions of patients before and after treatment showed that the mean ECOG scores of advanced GC patients in the simple group before and after treatment were 1.28±0.18 and 1.11±0.20, respectively, although the difference was not statistically significant (Z=−0.958, P>0.05), indicating that the effect on the improvement in the physical conditions of this group of patients after treatment was minimal. The mean ECOG scores before and after treatment in the combination group were 1.67±0.16 and 1.13±0.17, respectively, and the difference was statistically significant (Z=−2.480, P<0.05), indicating that the physical condition of patients in this group improved significantly after treatment. The main reason for the significant improvement in the physical conditions of advanced GC patients in the combination group should also involve the effective control of local malignant ascites by IPC, which functions to promote better rehabilitation of the body. The incidence of side effects in the simple group was slightly lower than that in the combination group; however, the difference between these two groups was not statistically significant (P>0.05). Neither of the groups experienced adult respiratory distress syndrome caused by IPC when using mitomycin C, as reported by Alonso et al. (23). Ceelen et al. (24) performed IPC using oxaliplatin, which caused severe complications, such as haemorrhagic peritonitis. Therefore, this retrospective study and previous relevant studies have indicated that although traditional IPC did not have further enhance chemotherapy efficacy, as with intraperitoneal hyperthermic perfusion chemotherapy, the IPC still played an active role in newly diagnosed GC combined with malignant ascites patients, in either the local function of ascites or the systemic control of tumour lesions in combination with intravenous chemotherapy.
In conclusion, our study shows IPC was safe, reliable, and effective; it significantly improved the natural disease courses of patients, relieved pain and suffering, and prolonged the patients’ lives. Additionally, functional imaging of GC may define tumor biological characteristics (25-28), and help therapeutic planning with personalized medicine (29). Therefore, IPC is an excellent strategy for the treatment of GC combined with malignant ascites, and its mechanism of action is worthy of further study (30).
Acknowledgments
Funding: This study was supported by grants from Natural Science Foundation of Fujian Province (No. 2016J01546) and Critical Patented Project of The Science & Technology Bureau of Fujian Province (No. 2013YZ0002-2).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Yì-Xiáng J. Wáng, Yong Wang) for the series “Translational Imaging in Cancer Patient Care” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.11.22). The series “Translational Imaging in Cancer Patient Care” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval for the study was provided by the Ethics Committee of Fujian Medical University Union Hospital (Ref. No. 2017KY082). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Maeda H, Kobayashi M, Sakamoto J, et al. Evaluation and treatment of malignant ascites secondary to gastric cancer. World J Gastroenterol 2015;21:10936-47. [Crossref] [PubMed]
- Kitayama J, Ishigami H, Yamaguchi H, et al. Salvage gastrectomy after intravenous and intraperitoneal paclitaxel (PTX) administration with oral S-1 for peritoneal dissemination of advanced gastric cancer with malignant ascites. Ann Surg Oncol 2014;21:539-46. [Crossref] [PubMed]
- Cao H, Bian Y, Zhao G, et al. Further recognition and improvement of multimodality treatment for advanced gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2014;17:1051-9. [PubMed]
- Ammouri L, Prommer EE. Palliative treatment of malignant ascites: profile of catumaxomab. Biologics 2010;4:103-10. [PubMed]
- Adam RA, Adam YG. Malignant ascites: past, present, and future. J Am Coll Surg 2004;198:999-1011. [Crossref] [PubMed]
- Liang H. Intraperitoneal slow released chemotherapy for advanced gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2012;15:116-7. [PubMed]
- Dedrick RL, Myers CE, Bungay PM, et al. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep 1978;62:1-11. [PubMed]
- Kuzuya T, Yamauchi M, Ito A, et al. Pharmacokinetic characteristics of 5-fluorouracil and mitomycin C in intraperitoneal chemotherapy. J Pharm Pharmacol 1994;46:685-9. [Crossref] [PubMed]
- Fushida S, Furui N, Kinami S, et al. Pharmacologic study of intraperitoneal paclitaxel in gastric cancer patients with peritoneal dissemination. Gan To Kagaku Ryoho 2002;29:2164-7. [PubMed]
- Ceelen WP, Hesse U, de Hemptinne B, et al. Hyperthermic intraperitoneal chemoperfusion in the treatment of locally advanced intra-abdominal cancer. Br J Surg 2000;87:1006-15. [Crossref] [PubMed]
- Topuz E, Basaran M, Sai P, et al. Adjuvant intraperitoneal chemotherapy with cisplatinum, mitoxantrone, 5-fluorouracil and calcium folinate in patients with gastric cancer: A phase II study. Am J Clin Oncol 2002;25:619-24. [Crossref] [PubMed]
- Pestieau SR, Schnake KJ, Stuart OA, et al. Impact of carrier solutions on pharmacokinetics of intraperitoneal chemotherapy. Cancer Chemother Pharmacol 2001;47:269-76. [PubMed]
- Marutsuka T, Shimada S, Shiomori K, et al. Mechanisms of peritoneal metastasis after operation for non-serosa-invasive gastric carcinoma: an ultrarapid detection system for intraperitoneal free cancer cells and a prophylactic strategy for peritoneal metastasis. Clin Cancer Res 2003;9:678-85. [PubMed]
- Wang J, Chen J, Ma S. Preoperative intraperitoneal versus intravenous carboplatin chemotherapy for advanced gastric cancer, pharmacokinetics and drug accumulation study. Zhonghua Zhong Liu Za Zhi 1997;19:300-2. [PubMed]
- Nakamura R, Saikawa Y, Kubota T, et al. Preoperative combination chemotherapy with TS-1 is effective in a case gastric cancer with peritoneal dissemination. Gan To Kagaku Ryoho 2005;32:1323-6. [PubMed]
- Kobayashi M, Sakamoto J, Namikawa T, et al. Pharmacokineticstudy of paclitaxel in malignant ascites from advanced gastriccancer patients. World J Gastroenterol 2006;12:1412-5. [Crossref] [PubMed]
- Kodera Y, Ito Y, Ito S, et al. Intraperitoneal paclitaxel: a possible impact of regional delivery for prevention of peritoneal carcinomatosis in patients with gastric carcinoma. Hepatogastroenterology 2007;54:960-3. [PubMed]
- Shimoyama S, Kaminishi M. A three-year survivor case of gastric cancer with peritoneal dissemination--an outpatient with second-line weekly paclitaxel. Gan To Kagaku Ryoho 2008;35:1003-7. [PubMed]
- Ishigami H, Kitayama J, Kaisaki S, et al. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol 2010;21:67-70. [Crossref] [PubMed]
- Yamaguchi H, Kitayama J, Ishigami H, et al. A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with S-1 for treatment of gastric cancer with macroscopic peritoneal metastasis. Cancer 2013;119:3354-8. [Crossref] [PubMed]
- Alonso O, Sugarbaker PH. Adult respiratory distress syndrome occurring in two patients undergoing cytoreductive surgery plus perioperative intraperitoneal chemotherapy: case reports and a review of the literature. Am Surg 2000;66:1032-6. [PubMed]
- Ceelen WP, Peeters M, Houtmeyers P, et al. Safety and efficacy of hyperthermic intraperitoneal chemoperfusion with high-dose oxaliplatin in patients with peritoneal carcinomatosis. Ann Surg Oncol 2008;15:535-41. [Crossref] [PubMed]
- Liu S, Zheng H, Pan X, et al. Texture analysis of CT imaging for assessment of esophageal squamous cancer aggressiveness. J Thorac Dis 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Yuan J, Wong OL, Lo GG, et al. Statistical assessment of bi-exponential diffusion weighted imaging signal characteristics induced by intravoxel incoherent motion in malignant breast tumors. Quant Imaging Med Surg 2016;6:418-29. [Crossref] [PubMed]
- Yan C, Pan X, Chen G, et al. A pilot study on correlations between preoperative intravoxel incoherent motion MR imaging and postoperative histopathological features of rectal cancers. Transl Cancer Res 2017;6:1050-60.
- Yuan J, Lo G, King AD. Functional magnetic resonance imaging techniques and their development for radiation therapy planning and monitoring in the head and neck cancers. Quant Imaging Med Surg 2016;6:430-48. [Crossref] [PubMed]
- Wang YX, Lin J. Preface to 2017 focused issue: Quantitative Imaging of Thoracic Diseases. J Thorac Dis 2017;9:4723. [Crossref] [PubMed]
- Wang ZM, Zhuang RY, Chen Y, et al. A pilot study of chemotherapy combined with intraperitoneal perfusion of cytokine-induced killer cells for advanced gastric cancer patients with ascites. Zhonghua Wei Chang Wai Ke Za Zhi 2013;16:28-31. [PubMed]


