
Head-to-head comparison of serum and urine cytokeratin-19 fragments (CYFRA 21–1) for bladder cancer diagnosis
Introduction
Bladder cancer is the ninth leading cause of death in males. It is estimated that 429,800 new cases were diagnosed and 165,100 deaths occurred in 2012 worldwide (1). The prognosis of bladder cancer is poor, especially for patients at advanced stage (2). The patient outcomes can be improved if the diagnosis can be established in a timely and accurate manner. Currently, the cystoscopy or voided urine cytology (VUC) are gold standards for bladder cancer diagnosis. However, cystoscopy is an expensive, invasive and uncomfortable tool. In addition, the diagnostic accuracy of cystoscopy is largely affected by the experience of the operator. VUC has a high diagnostic specificity for bladder cancer; however, its sensitivity is only 0.37Jeny (3). Therefore, it is of great value to diagnose bladder cancer using non-invasive biomarkers (4).
Over the past years, many serum or urine biomarkers have been identified (5), such as nuclear matrix protein 22 (NMP22), qualitative or quantitative bladder tumour antigen (BTA) and cytokeratin-19 fragment (CYFRA 21-1). Among the available biomarkers, CYFRA 21-1 is promising, as a meta-analysis shows that the area under the summarized receiver operating characteristic (sROC) for the serum and urine CYFRA 21-1 are 0.88 and 0.87, respectively (6). To the best of our knowledge, no previous studies have compared the diagnostic accuracy of serum and urine CYFRA 21-1 in a head-to-head manner. Therefore, we performed a head-to-head comparison study to (I) compare the diagnostic accuracy of serum and urine CYFRA 21-1 for bladder cancer and (II) investigate whether the diagnostic accuracy can be improved if the serum and urine CYFRA 21-1 are used together.
Methods
Subjects
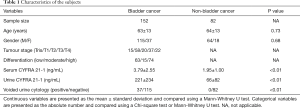
This is a prospective study performed between January 1, 2016 and December 31, 2016, in Daping Hospital. The study cohort includes 152 bladder cancer patients and 82 controls. All the subjects were suspected of bladder cancer and have received cystoscopy to determine if the patient had bladder cancer. Among the 82 controls, 40 had cystitis, 15 had urolithiasis, 14 had a urinary tract infection, 7 had kidney carcinomas and 6 had a benign bladder tumour.
The serum and random urine samples were obtained from the subjects within 24 hours after admission. CYFRA 21-1 levels in the serum and urine were measured within 24 hours after collection. Both the serum and urine CYFRA 21-1 were detected using a Maglumi 2000 Plus immunoassay analyser (Shenzhen, China). Test results of both the serum and urine CYFRA 21-1 were blinded to the clinician. Clinical details of the subjects were blinded to the technicians who were responsible for the CYFRA 21-1 determination.
This study was approved by the Ethics Committee of Daping Hospital (NO: 52). All the included subjects or their legal representatives provided informed consent.
Statistical analysis
Because the continuous data in this study were not normally distributed (tested by Kolmogorov-Smirnov test), we used a Mann-Whitney test or Kruskal-Wallis test to compare the continuous variables. Dunn’s post hoc procedure was used for multiple comparisons and the significance level of the test was set at 0.05/n, where n represents the number of pairwise comparisons. Categorical data were compared using a Chi-square test. The diagnostic value of the serum and urine CYFRA 21-1 were assessed using a ROC curve analysis. A multivariable logistic regression model was constructed to combine the serum and urine CYFRA 21-1 as a single indicator. The threshold with the maximum Youden index was set as the optimal threshold and the corresponding sensitivity and specificity were calculated. The areas under the ROC curves (AUCs) were compared using Delong’s approach (7). All analyses were performed in SPSS 18.0 (SPSS Inc., Chicago, IL, USA) and Sigmaplot 12.0 (Systat Software, Inc., San Jose, CA, USA). A P value less than 0.05 was defined as statistically significant.
Results
Characteristics of the subjects
Table 1 lists the characteristics of the subjects. Age and gender were comparable between the subjects with or without bladder cancer. Significantly higher serum and urine CYFRA 21-1 were observed in patients with bladder cancer (P<0.01).
Full table
Association between CYFRA 21-1 and clinical characteristics
As shown in Figure 1, both the serum and urine CYFRA 21-1 were significantly increased with advanced of tumour stage and differentiation (P<0.05 for both). If the Tris stage was excluded from the analysis, the urine CYFRA 21-1 was significantly increased as the stage advanced (P<0.01), but we failed to observe a significant difference in the serum CYFRA 21-1 between the T1 stage and the T4 stage (P=0.08). For the post hoc analysis, only the following pairs of comparisons were statistically significant: urine CYFRA 21-1 and stage, Tris vs. T2, Tris vs. T3, Tris vs. T4, T1 vs. T2, and T1 vs. T3, and in terms of the urine CYFRA 21-1 grade: low vs. high and moderate vs. high. Using a Spearman’s correlation analysis, we found that both the serum and urine CYFRA 21-1 were positively correlated with tumour stage and differentiation (P<0.05 for both). Bladder cancer patients with positive VUC had significantly higher urine CYFRA 21-1 (P<0.01), but serum CYFRA 21-1 differences in patients with positive or negative VUC were not statistically significant (P=0.12).

Diagnostic accuracy of serum and urine CYFRA 21-1 for bladder cancer
Figure 2 is an ROC curve describing the diagnostic accuracy of both the serum and urine CYFRA 21-1 for bladder cancer. The AUCs (95% CI) for serum and urine CYFRA 21-1 were 0.74 (0.68–0.81) and 0.74 (0.68–0.80), respectively. The differences between the AUCs of serum and urine CYFRA 21-1 were not statistically significantly (P=0.91). The AUC (95% CI) for the combination of serum and urine CYFRA 21-1 was 0.83 (0.78–0.88), which was significantly higher than that of serum (P=0.04) and urine (P=0.03) CYFRA 21-1 alone.
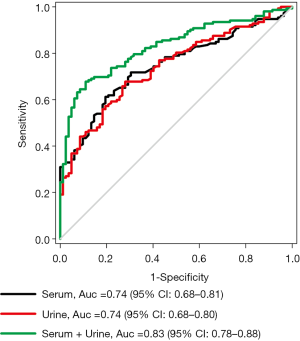
Table 2 lists the thresholds, as well as the corresponding sensitivities and specificities, of both the serum and urine CYFRA 21-1.

Full table
Discussion
In this study, we compared the diagnostic value of the serum and urine CYFRA 21-1 for bladder cancer and found that (I) the diagnostic accuracy of both the serum and urine CYFRA 21-1 was fair, as their AUCs were only 0.74; (II) both the serum and urine CYFRA 21-1 increased with advanced tumour stage, indicating that they are potential prognostic factors for bladder cancer; and c) the diagnostic value of serum and urine CYFRA 21-1 was comparable, and combinational use can improve the diagnostic accuracy of bladder cancer.
Although many studies have investigated the diagnostic accuracy of serum or urine CYFRA 21-1 for bladder cancer (8-12), our study shows its strengths. First, to the best of our knowledge, this is the first study to do a head-to-head comparison of the diagnostic accuracy of CYFRA 21-1 in a cohort. Second, this is a double-blind study. The laboratory technicians were blinded to the clinical details of the subjects and the clinicians who made the diagnosis were blinded to the serum and urine CYFRA 21-1 test results. Therefore, incorporation bias and review bias were avoided (13). Third, all subjects in this study received cystoscopy. Therefore, partial verification bias is avoided (13,14). Fourth, all the subjects enrolled in this study had signs or symptoms of bladder cancer. Therefore, the study cohort had good representativeness.
Because the sensitivity and specificity are greatly affected by the threshold that was adopted, both indicators have limitations in estimating the overall diagnostic accuracy of an index test. It is widely accepted that AUC is a global indicator that reflects the overall diagnostic accuracy of an index test (15). The AUC ranges from 0.5 to 1, with a higher value indicating a stronger diagnostic performance. In this study, we found that AUCs for both the serum and urine CYFRA 21-1 were 0.71, indicating that the diagnostic accuracy of the serum and urine CYFRA 21-1 was fair. The difference between the AUCs for serum and urine CYFRA 21-1 was not significant, indicating that the diagnostic accuracy of the serum and urine CYFRA 21-1 was comparable. Our results were consistent with a previous meta-analysis (6), which reported that the areas under the summary ROC curve for serum and urine CYFRA 21-1 were 0.88 and 0.87, respectively. The AUCs in our study were lower than those reported by some previous studies (10,11). This inconsistency may be due to differences in the disease spectrum and disease prevalence of the cohorts. It is well known that the AUC of an index test is greatly affected by the disease spectrum and prevalence of the studied cohort (16,17).
We used a logistic regression model to incorporate the serum and urine CYFRA 21-1 into a model. ROC curve analysis was used to estimate the diagnostic accuracy of this model. We found that the AUC of the model was 0.83, which was significantly higher than that of the serum and urine CYFRA 21-1. This result indicates that using the serum and urine CYFRA 21-1 together can improve the diagnostic accuracy of bladder cancer. Therefore, for a suspicious bladder cancer patient, both the serum and urine CYFRA 21-1 should be determined to improve the diagnostic accuracy of bladder cancer.
Taken together, our study indicated that the diagnostic accuracy of the serum and urine CYFRA 21-1 was comparable and that using them together can improve the diagnostic accuracy of bladder cancer. Due to the small sample size and single-centre design, further studies with larger sample sizes are needed to further estimate the diagnostic accuracy of serum and urine CYFRA 21-1.
Acknowledgments
Funding: This work was supported by the International Cooperation in Science and Technology of the Science and Technology Ministry (No. 2014DFR30860).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.01.08). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Daping Hospital (NO: 52). All the included subjects or their legal representatives provided informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Kamat AM, Hahn NM, Efstathiou JA, et al. Bladder cancer. Lancet 2016;388:2796-810. [Crossref] [PubMed]
- Xie Q, Huang Z, Zhu Z, et al. Diagnostic Value of Urine Cytology in Bladder Cancer. A Meta-Analysis. Anal Quant Cytopathol Histpathol 2016;38:38-44. [PubMed]
- Kojima T, Kawai K, Miyazaki J, et al. Biomarkers for precision medicine in bladder cancer. Int J Clin Oncol 2017;22:207-13. [Crossref] [PubMed]
- Chou R, Gore JL, Buckley D, et al. Urinary Biomarkers for Diagnosis of Bladder Cancer: A Systematic Review and Meta-analysis. Ann Intern Med 2015;163:922-31. [Crossref] [PubMed]
- Huang YL, Chen J, Yan W, et al. Diagnostic accuracy of cytokeratin-19 fragment (CYFRA 21-1) for bladder cancer: a systematic review and meta-analysis. Tumour Biol 2015;36:3137-45. [Crossref] [PubMed]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837-45. [Crossref] [PubMed]
- Stieber P, Dienemann H, Hasholzner U, et al. Comparison of CYFRA 21-1, TPA and TPS in lung cancer, urinary bladder cancer and benign diseases. Int J Biol Markers 1994;9:82-8. [PubMed]
- Pariente JL, Bordenave L, Michel P, et al. Initial evaluation of CYFRA 21-1 diagnostic performances as a urinary marker in bladder transitional cell carcinoma. J Urol 1997;158:338-41. [Crossref] [PubMed]
- Pariente JL, Bordenave L, Jacob F, et al. Analytical and prospective evaluation of urinary cytokeratin 19 fragment in bladder cancer. J Urol 2000;163:1116-9. [Crossref] [PubMed]
- Jeong S, Park Y, Cho Y, et al. Diagnostic values of urine CYFRA21-1, NMP22, UBC, and FDP for the detection of bladder cancer. Clin Chim Acta 2012;414:93-100. [Crossref] [PubMed]
- Gkialas I, Papadopoulos G, Iordanidou L, et al. Evaluation of urine tumor-associated trypsin inhibitor, CYFRA 21-1, and urinary bladder cancer antigen for detection of high-grade bladder carcinoma. Urology 2008;72:1159-63. [Crossref] [PubMed]
- Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25. [Crossref] [PubMed]
- de Groot JA, Bossuyt PM, Reitsma JB, et al. Verification problems in diagnostic accuracy studies: consequences and solutions. BMJ 2011;343:d4770. [Crossref] [PubMed]
- Linnet K, Bossuyt PM, Moons KG, et al. Quantifying the Accuracy of a Diagnostic Test or Marker. Clin Chem 2012;58:1292-301. [Crossref] [PubMed]
- Schmidt RL, Factor RE. Understanding sources of bias in diagnostic accuracy studies. Arch Pathol Lab Med 2013;137:558-65. [Crossref] [PubMed]
- Whiting P, Rutjes AW, Reitsma JB, et al. Sources of variation and bias in studies of diagnostic accuracy: a systematic review. Ann Intern Med 2004;140:189-202. [Crossref] [PubMed]


