
Docetaxel-polymeric nanoparticle enhances radiotherapeutic efficacy in human pancreatic cancer
Introduction
Pancreatic cancer is a lethal cancer of which 5-year survival rate is less than 5% (1,2). Almost all of pancreatic cancer patients with the diagnosis of pancreatic ductal adenocarcinoma (PDAC) show notoriously poor prognosis and eventually almost all patients die from the disease (3,4). Several chemotherapy drugs such as gemcitabine (GEM), paclitaxel (PTX), and 5-fluorouracil are effective in the treatment of pancreatic cancer, and even several diagnosis and treatments have recently progressed, but the survival effect is still negligible (5,6). Therefore, it is urgently necessary to develop new therapeutic means for the majority of patients with pancreatic cancer.
The taxanes have been widely used in cytotoxic treatment of many solid tumors as a family of very efficient anticancer drugs (7,8). Docetaxel (DTX) has been known to be superior to PTX in clinical efficacy, but it exhibits severe side effects (9,10). Taxotere, the most famous commercial formulation of DTX contains the non-ionic surfactant Tween 80 (polysorbate 80) and 13% ethanol; the side effects caused by DTX and the solvent have significantly limited its clinical use (11). We have developed DTX-polymeric nanoparticle (DTX-PNP) in which DTX was incorporated to reduce the side effects and improve the therapeutic efficacy, and recently finished its phase 1 clinical study. In our previous report, the result of the phase 1 clinical trial of DTX-PNP including side-effect profile, pharmacokinetics, and document any observed antitumor activity was introduced (9,12).
Radiation therapy has been considered as an important treatment for various cancer treatments. Radiotherapy plays a role especially in local progress pancreatic cancer when patients have no distant disease after chemotherapy of several months (13,14). However, not all cancers respond well to radiation therapy. Increasing the radiation dose cannot be an alternative to dose enhancement because it severely damages normal tissue around the tumor, increases the possibility of wound complications and changes the cells to radiation resistance (15,16). In order to maximize the efficacy of radiotherapy, it should be aimed to simultaneously increase the killing effect on tumor tissues and reduce side effects on normal tissues. In this study, the property of DTX-PNP that was formulated to improve chemoradiotherapeutic efficacy and reduce side effects was investigated for the enhancement of radiotherapy response in pancreatic cancer for further clinical application.
Methods
Clonogenic survival assay
To evaluate the drug efficacy in cell proliferation and survival, cells were plated in a 6-well tissue culture plate at a density of 5×104 cells/well. Cells were exposed to 2 Gy of ionizing radiation (IR) in the presence of drugs such as Taxotere, Gemzar, DTX-PNP (1 nM, 5 nM, 1 nM, respectively), and incubated for 24 h in the presence of drugs. After withdrawal of drugs from medium, cells were incubated for approximately 10 days to form colonies. Cells were stained with 0.5% crystal violet (Sigma-Aldrich, St Louis, MO), and the colony forming rate was calculated using Image J software.
Western blot analysis
BxPC-3 cells treated with drugs for 24 or 48 h were harvested, washed twice with PBS, and lysed with cell lysis reagent [no. R4100-010; RIPA cell lysis buffer (1×) with EDTA, GenDEPOT] on ice. Primary antibody against cleaved caspase-3 (no. 9661; Cell Signaling Technology, Inc.) was used at 1:1,000 dilution, and HRP-conjugated secondary antibody was used at 1:2,000 dilution in 5% skim milk. After final washing, the membrane was exposed to an enhanced ECL solution and chemiluminescence image was acquired using ImageQuant LAS 4000 (GE Healthcare Life Sciences).
Immunohistochemical analysis
Tumor tissue isolated from mouse was fixed in 4% paraformaldehyde overnight, permeabilized with 70% ethanol overnight. Paraffin-embedded tumor tissue was sectioned in 3 µm, mounted on silane-coated slides. The tissue was blocked with 5% bovine serum albumin (NGS) in PBST (0.01% Triton X-100 in PBS) and incubated with anti-cleaved caspase-3 (1:1,000; no. 9661; Cell Signaling Technology, Inc.) and anti-alpha-tubulin (1:200; no. 2125; Cell Signaling Technology, Inc.) diluted in PBST overnight at 4 °C, the tissue for cleaved caspase-3 was incubated with anti-rabbit IgG-HRP (1:2,000; Jackson ImmunoResearch Laboratories, Inc.) for 1 h at room temperature and visualized with DAB (vector SK 4100). The tissue for alpha-tubulin was incubated with Alexa Fluro® 488 donkey anti-rabbit (1:500; Jackson ImmunoResearch Laboratories, Inc.) for 2 h at room temperature and visualized with VECTASHIELD Mounting Media containing DAPI (Vector Laboratories).
Tumor growth inhibition and survival fraction
All animal experiments were performed following the protocol approved by the Institutional Animal Care and Use Committee of the Asan Institute for Life Sciences. AsPC-1 or BxPC-3 cells-derived xenograft tumor model and patient-derived xenograft (PDX) tumor model made of male athymic nude mice (BALB/c nu/nu; 6 weeks old; Japan SLC, Hamamatsu, Japan) was used for the examination of in vivo therapeutic efficacy. To produce the xenograft model, suspension of 1×106 cells and 3 mm3 tissues was implanted subcutaneously (s.c) into a right hind leg of mice. Tumors were measured by length, width and tumor volume was calculated as (length × width2) ×0.5. Mice were started to receive treatment when the average tumor volume reached 80 to 120 mm3. Taxotere and DTX-PNP at 10 mg/kg and Gemzar at 50 mg/kg were intravenously (i.v.) administrated through tail vein at 24 h after 5 Gy IR that was locally delivered to tumor using a 6-MV photon beam linear accelerator (CL/1800, Varian Medical System, Palo Alto, CA, USA).
Results
Radiosensitization effect of DTX-PNP in pancreatic cells
To examine radiosensitization effect of DTX-PNP, clonogenic assay survival was done with human pancreatic cancer BxPC-3 cells exposed to Taxotere, Gemzar and DTX-PNP combined with 2 Gy IR. The number of clonies were much decreased by DTX-PNP and IR treatment (Figure 1). Quantificated result showed that the most effective radiosensitization was displayed by treatment of DTX-PNP and IR (Figure 1B). Combination group of IR and DTX-PNP, as well as IR and Taxotere, markedly increased cleaved caspase-3 as compared to control and IR alone (Figure 1C), suggesting that combination therapy of IR and DTX induced apoptosis higher than IR and Gemzar treatment groups. These results indicated that DTX-PNP displayed strong radiosensitization effect in human pancreatic cancer cells.
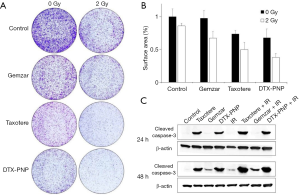
Induction of apoptosis and tubulin polymerization in xenograft tumor tissue
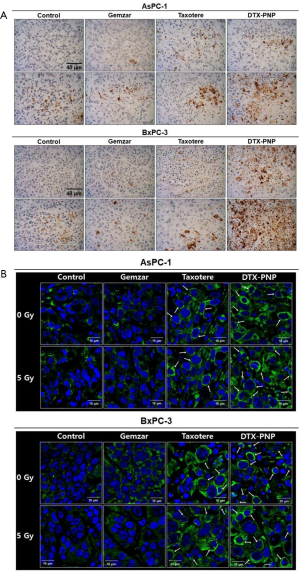
To assess whether combination treatment of DTX-PNP and IR induce apoptosis and tubulin polymerization in vivo, human pancreatic cancer cell-derived xenograft tumor mouse was treated and the tumor tissue was stained for cleaved caspase-3 and α-tubulin. DTX-PNP and IR induced significantly potentiated apoptosis (Figure 2A), suggesting that DTX-PNP exhibited great radiosensitization effect in vivo. The ability of taxanes to bind and polymerize tubulin has been known to display anti-cancer effect. The higher the level of tubulin polymerization, the better the anti-cancer effect. In the result of tubulin staining, DTX-PNP and IR displayed effective polymerization of tubulin as likely or more than Taxotere and IR (Figure 2B), indicating that DTX-PNP preserved the potential of DTX and exerted the action mechanism to the in vivo tumor tissue. These results strongly suggested that DTX-PNP could be an effective radiosensitizer in vivo.
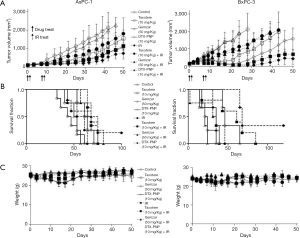
Enhanced radiotherapeutic efficacy and improved survival by DTX-PNP in various xenograft models
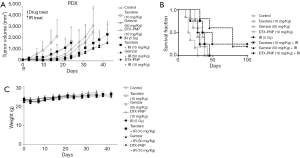
To evaluate the effectiveness of DTX-PNP to enhance in vivo radiotherapeutic efficacy, the inhibition of tumor growth was examined in mice bearing AsPC-1 or BxPC-3 derived tumors. In both xenograft tumor models, DTX-PNP and IR treated group showed remarkably enhanced tumor growth inhibition compared to IR group, which was greater than that of Taxotere and IR or Gemzar and IR (Figure 3A). Mice treated with Gemzar that was the representative drug for pancreatic cancer didn’t show any anti-cancer effect in these models, while Taxotere exhibited notable anti-cancer effect. Nano-particulated DTX-PNP containing DTX as active pharmaceutical ingredient was shown greater anti-cancer effect than that of free DTX, which might be due to long circulating and passive targeting. In the same context, DTX-PNP displayed the most effectively enhanced radiotherapeutic efficacy beyond Taxotere or Gemzar. Survival fraction for control, Gemzar, Taxotere or DTX-PNP treated groups of AsPC-1 were 61, 57, 71 and 75 days, respectively (Figure 3B). For BxPC-3, control, Gemzar, Taxotere and DTX-PNP treated groups were 35, 24, 39 and 65 days, respectively. By the combination of IR, the survivals of control, Gemzar, Taxotere were prolonged to 64, 81, 75 days in AsPC-1, and 46, 39, 85 days in BxPC-3. Importantly, the DTX-PNP combination with IR provided a substantial survival benefit that was prolonged to more than 100 days in AsPC-1, or more than 120 days in BxPC-3. There was no observable body weight loss in the mice during whole experimental period (Figure 3C). These results strongly suggested that DTX-PNP greatly enhanced radiotherapeutic efficacy in animal models derived with human pancreatic cancer cells. For further evaluation of effectiveness of DTX-PNP on radiotherapy for pancreatic cancer treatment, a case of pancreatic cancer PDX model was examined. In result, DTX-PNP-treated group showed the most potent radiosensitization effect which was greater than Taxotere and similar to Gemzar (Figure 4A). Survival fraction for control, Taxotere, Gemzar and DTX-PNP treated group of PDX were 28, 38, 42 and 45 days, respectively. When combined with IR, the survival fraction of DTX-PNP significantly prolonged (Figure 4B). A change of body weight was not observed in any group, indicating that the treatments did not induce a severe toxicity (Figure 4C).
Discussion
For the treatment of advanced pancreatic cancer, GEM is one of standard care (17), but large portion of patients often respond poorly to this agent. As an alternative, radiotherapy is routinely applied to patients whose tumor is neither responding to GEM nor able to be subjected to surgical intervention. Pancreatic cancer commonly encompasses major vessels named superior mesenteric artery of which circumferential contact with tumor is often a contraindication to surgery. Radiotherapy could be a solution to provide a good benefit through shrinking the circumferential contact of the vessels to allow surgical intervention, in addition to its therapeutic effect on cancer cells through induction of DNA damage leading to cell death. However, not all tumors respond well to radiotherapy, and sometimes radiotherapy could induce damages unexpectedly in healthy adjacent tissues. To overcome the current limitations in pancreatic cancer, we have tried in this study to apply DTX-PNP for the improvement of chemoradiotherapeutic efficacy and reduce adverse side effect.
This study demonstrated that DTX-PNP showed a clinically applicable effectiveness as a radiosensitizer for human pancreatic cancer. While DTX has not been approved for pancreatic cancer yet, it was proved in this study that nanoparticulated DTX showed a great efficacy in animal models of pancreatic cancer, especially in combination with radiotherapy. DTX is a well-established chemotherapeutic agent already widely used in various types of cancer, but has an obvious limit caused by toxicity in expanding the scope of application. In order to overcome the limitation of toxicity and simultaneously to maximize therapeutic effect, we have manufactured DTX-PNP (11). The excellent anti-cancer effect of DTX-PNP was confirmed through non-clinical studies (9), and the safety of human was verified by phase I clinical study (12). In this study, we examined the effects of DTX-PNP as a concurrent treatment with radiotherapy on pancreatic cancer, the most difficult cancer to treat. The results of this study clearly indicate that DTX-PNP can be applied to the treatment of pancreatic cancer and that rapid development and clinical feasibility will be possible because of the advantages of nanomedicine.
Number of nanomedicine has been recently developed in various cancer types, including pancreas cancer (8). Nanoalbumin bound PTX (Abraxane) has been approved in metastatic pancreatic cancer in combination with Gemzar (6). Liposome-incorporated irinotecan (Onivyde) has been approved and shown an effect in pancreatic cancer in combination with 5-fluorouracil (5-FU) (18). Although nanoparticles have begun to be applied to pancreatic cancer, no nanomedicinal drug has yet been developed as a combination therapy for radiation therapy. This study suggests that DTX-PNP is a highly promising drug for radiation therapy to treat human pancreatic cancer and expected to be the fastest clinical application.
Acknowledgments
Funding: The present study was supported by grants from the Korean Health Technology R & D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry for Health & Welfare, Republic of Korea (HI06C0868 and HI15C0972); the National R & D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (15201101); and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1D1A1B03034359, NRF-2017R1D1A1B03035167 and NRF-2017R1D1A1B03034857).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.01.09). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Animal Care and Use Committee of the Asan Institute for Life Sciences (No. 2017-12-026) in compliance with the national and institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gonzalez-Villasana V, Rodriguez-Aguayo C, Arumugam T, et al. Bisphosphonates inhibit stellate cell activity and enhance antitumor effects of nanoparticle albumin-bound paclitaxel in pancreatic ductal adenocarcinoma. Mol Cancer Ther 2014;13:2583-94. [Crossref] [PubMed]
- Tuli R, Surmak AJ, Reyes J, et al. Radiosensitization of Pancreatic Cancer Cells In Vitro and In Vivo through Poly (ADP-ribose) Polymerase Inhibition with ABT-888. Transl Oncol 2014; [Epub ahead of print]. [Crossref] [PubMed]
- Al-Assar O, Bittner MI, Lunardi S, et al. The radiosensitizing effects of Nelfinavir on pancreatic cancer with and without pancreatic stellate cells. Radiother Oncol 2016;119:300-5. [Crossref] [PubMed]
- Kudgus RA, Szabolcs A, Khan JA, et al. Inhibiting the growth of pancreatic adenocarcinoma in vitro and in vivo through targeted treatment with designer gold nanotherapeutics. PLoS One 2013;8:e57522 [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Ernsting MJ, Tang WL, MacCallum NW, et al. Preclinical pharmacokinetic, biodistribution, and anti-cancer efficacy studies of a docetaxel-carboxymethylcellulose nanoparticle in mouse models. Biomaterials 2012;33:1445-54. [Crossref] [PubMed]
- Louage B, De Wever O, Hennink WE, et al. Developments and future clinical outlook of taxane nanomedicines. J Control Release 2017;253:137-52. [Crossref] [PubMed]
- Jung J, Park SJ, Chung HK, et al. Polymeric nanoparticles containing taxanes enhance chemoradiotherapeutic efficacy in non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2012;84:e77-83. [Crossref] [PubMed]
- Woo HN, Chung HK, Ju EJ, et al. Preclinical evaluation of injectable sirolimus formulated with polymeric nanoparticle for cancer therapy. Int J Nanomedicine 2012;7:2197-208. [PubMed]
- Lee SW, Chang DH, Shim MS, et al. Ionically fixed polymeric nanoparticles as a novel drug carrier. Pharm Res 2007;24:1508-16. [Crossref] [PubMed]
- Song SY, Kim KP, Jeong SY, et al. Polymeric nanoparticle-docetaxel for the treatment of advanced solid tumors: phase I clinical trial and preclinical data from an orthotopic pancreatic cancer model. Oncotarget 2016;7:77348-57. [Crossref] [PubMed]
- Koshkina NV, Briggs K, Palalon F, et al. Autophagy and enhanced chemosensitivity in experimental pancreatic cancers induced by noninvasive radiofrequency field treatment. Cancer 2014;120:480-91. [Crossref] [PubMed]
- Tuli R, Surmak A, Reyes J, et al. Development of a novel preclinical pancreatic cancer research model: bioluminescence image-guided focal irradiation and tumor monitoring of orthotopic xenografts. Transl Oncol 2012;5:77-84. [Crossref] [PubMed]
- Brunner TB, Nestle U, Grosu AL, et al. SBRT in pancreatic cancer: what is the therapeutic window? Radiother Oncol 2015;114:109-16. [Crossref] [PubMed]
- Shirai Y, Shiba H, Iwase R, et al. Dual inhibition of nuclear factor kappa-B and Mdm2 enhance the antitumor effect of radiation therapy for pancreatic cancer. Cancer Lett 2016;370:177-84. [Crossref] [PubMed]
- Ottaiano A, Capozzi M, De Divitiis C, et al. Gemcitabine mono-therapy versus gemcitabine plus targeted therapy in advanced pancreatic cancer: a meta-analysis of randomized phase III trials. Acta Oncol 2017;56:377-83. [Crossref] [PubMed]
- Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 2016;387:545-57. [Crossref] [PubMed]


