
Clinical efficacies of gemcitabine combined with docetaxel single or plus bevacizumab as second-line therapy for malignant pleural mesothelioma
Introduction
Malignant pleural mesothelioma (MPM) is a rare malignant tumor originating from the pleural mesothelial tissue. There are significant differences in its incidence among different countries in the world: the incidence rate in China is about 0.3/100,000 to 0.5/100,000 (1), the incidence rate in the Netherlands is 1/100,000, while that in Australia is up to 4/100,000 (2). Histologically, MPM can be divided into the epithelium type, the sarcoma type, and the mixed type. Due to difficulties in early diagnosis, extremely high invasiveness, and lacking effective treatment, the prognosis is very poor, and the natural survival period is less than 1 year. At present, treatments against advanced MPM are based on pemetrexed combined with platinum-based chemotherapy (3-6), but the effect is not ideal. Until 2016, the three-stage clinical MAPS study of chemotherapy-based combination with bevacizumab, one anti-vascular drug, confirmed that (7) standard pemetrexed combined with cisplatin alone can extend the median survival period from 16.1 to 18.8 months than that combined with bevacizumab. At present, chemotherapy combined with bevacizumab has been written into the NCCN guidelines (8). However, second-line chemotherapy against MPM still has no standard treatment program currently, and the recommended drugs include gemcitabine, vinorelbine, or docetaxel (9-13), which can be used alone or in combination. However, literature data reveal that for patients with better PS scores, the chemotherapy regimen of two-drug combination can achieve better effect than single-drug chemotherapy (12,13). Our study was designed to confirm the efficacies of gemcitabine combined with docetaxel in treating MPM, and to further explore whether the two-drug chemotherapy-based combination with anti-vascular therapy can further improve the efficacies.
Methods
Patient selection and general information
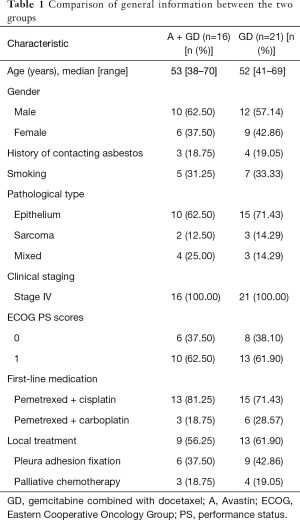
The clinical data of 37 MPM patients treated in the First Affiliated Hospital of Guangzhou Medical University and applied at least two treatment cycles of gemcitabine combined with docetaxel, together with bevacizumab or not, from March 1, 2010 to March 1, 2016 were retrospectively collected. All the patients were clearly diagnosed as MPM (clinical stage IV) by pathologic evidence, and had complete imaging data for efficacy evaluation. The first-line treatment was the standard protocol of pemetrexed combined with cisplatin or carboplatin. There were 19 males and 18 females, age from 38 to 70 years, with the median age as 52 years. According to the TNM staging system of MPM issued by the Union for International Cancer Control (UICC, seventh edition), all these 37 patients were classified into clinical stage IV (Table 1). This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Guangzhou Medical University [No. 2016(26)]. Written informed consent was obtained from all participants.
Full table
Grouping
A total of 21 patients were applied the protocol of gemcitabine combined with docetaxel (group GD), and 16 patients were further applied bevacizumab (group A + GD).
Treatment
Medicine include: Gemcitabine (Eli Lilly, USA, registration No. H20110535), Docetaxel (Docetaxel injection, Aventis, UK, registration No. H20090647), and bevacizumab (Avastin, Roche, Switzerland, registration No. JS20100049). The specific medication protocol was as follows: gemcitabine, 1,000 mg/m2 iv gtt, days 1–14; docetaxel, 60 mg/m2 iv gtt, days 1–14; one treatment course was 28 days; group A + GD: 7.5 mg/kg, days 1–14, until the disease progressed or up to six courses.
Criteria for evaluating efficacies
Referring to the RECIST (v 1.1), the outcomes were divided into complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD); the objective response rate (ORR) = ratio of the patients with CR + PR to the whole patients in the same group; the disease control rate (DCR) = ratio of the patients with CR + PR + SD to the whole patients in the same group. The long-term efficacy mainly recorded the progression free survival (PFS) time, which was the period from the start of applying the second-line medication to the progress or death. The overall survival (OS) time was the period from the start of applying the second-line medication to death or the final follow-up.
Criteria for evaluating adverse reactions
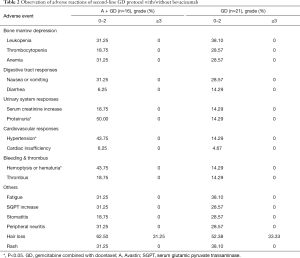
The adverse reactions were graded using the CTCAE (version 4.0, grade 0–4). The specific adverse reactions are shown in Table 2. No patient occurred adverse reaction-induced withdrawal during treatment. There was no significant difference in the bone marrow suppression, gastrointestinal adverse reactions, fatigue, alanine aminotransferase increase, stomatitis, peripheral neuritis, hair loss, rash, or other common chemotherapy toxicities between the two groups, but the incidence of proteinuria in group GD and A + GD were 14.29% and 50.00%, respectively (P=0.035), bleeding in groups GD and A + GD were 14.29% and 43.75%, respectively (P=0.048), and hypertension in groups GD and A + GD were 14.29% and 43.75%, respectively (P=0.048).
Full table
Statistical analysis
SPSS17.0 was used for the statistical analysis, the patients’ general information used the descriptive table; Pearson’s Chi-Square test was used to treatment efficacies (ORR, DCR) and adverse reactions between the two groups; randomization was stratified according to disease stage, Eastern Cooperative Oncology Group performance status (ECOG PS), sex etc.; the survival analysis used the Kaplan-Meier method, with the test level α=0.05.
Results
Evaluation of short-term efficacies
Among the 37 patients, 23 patients achieved the control of their disease conditions, including 1 case of CR, 9 cases of PR, 13 cases of SD, and 14 cases of PD, with ORR as 27.0%, DCR as 62.2%, and PFS as 4.5 months. The OS was 12.0 months. Further analysis showed that the ORR in groups GD and A + GD were 23.8% and 31.3%, respectively, χ2=1.255, P=0.145, and the difference was not statistically significant; the DCR in groups GD and A + GD were 52.4% and 75.0%, respectively, χ2=3.975, P=0.044, and the difference showed statistically significant. The short-term efficacy evaluation is shown in Table 3.

Full table
Evaluation of long-term efficacies
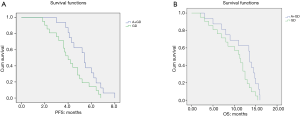
The long-term efficacy evaluation is shown in Table 3. The PFS of the 37 patients was 4.5 months, with OS as 12.0 months. Further analysis showed that the PFS in groups GD and A + GD were 4.0 and 5.4 months, respectively, χ2=4.615, P=0.032, and the difference was statistically significant. The survival curves of the two groups are shown in Figure 1A, with the OS as 11.3 and 13.6 months, respectively, χ2=4.484, P=0.028, and the difference was statistically significant. The survival curves are shown in Figure 1B.
Evaluation of adverse reactions
The specific adverse reactions are shown in Table 2. No patient occurred adverse reaction-induced withdrawal during treatment. There was no significant difference in the bone marrow suppression, gastrointestinal adverse reactions, fatigue, alanine aminotransferase increase, stomatitis, peripheral neuritis, hair loss, rash, or other common chemotherapy toxicities between the two groups, but the incidence of proteinuria in groups GD and A + GD were 14.29% and 50.00%, respectively (P=0.035), bleeding in groups GD and A + GD were 14.29% and 43.75%, respectively (P=0.048), and hypertension in groups GD and A + GD were 14.29% and 43.75%, respectively (P=0.048).
Discussion
MPM is a rare malignant tumor originating from the pleural mesothelial tissue. Histologically, MPM can be divided into the epithelium type, the sarcoma type, and the mixed type. Due to difficulties in early diagnosis, extremely high invasiveness, and lacking effective treatment, the prognosis is very poor, and the natural survival period is less than 1 year. At present, treatments against advanced MPM are based on pemetrexed combined with platinum-based chemotherapy (3-6), but the effect is not ideal. In 2003, Vogelzang et al. (3) reported that the third-stage clinical results of first-line pemetrexed combined with cisplatin or cisplatin alone, the median survival period was extended from 9.3 to 12.1 months, so that the chemotherapy of pemetrexed combined with cisplatin has been confirmed as the first-line standard chemotherapy protocol. However, due to cisplatin’s serious toxicities in the digestive tract and kidneys, its clinical applications have been limited to a certain limit. In 2006, Ceresoli et al. (4) firstly reported the second-stage clinical results of carboplatin, replacing cisplatin, in treating MPM and considered that pemetrexed combined with carboplatin for MPM has good tolerance, with the median survival time as 12.7 months, and the effect had no significant difference from the standard protocol of pemetrexed combined with cisplatin. Subsequently, Castagneto et al. (5) and Katirtzoglou et al. (6) also reported the two-stage clinical studies and had obtained the similar results. Therefore, in clinical practice, pemetrexed combined with carboplatin can also be used as the first-line standard protocol, especially for those MPM patients that can’t tolerate cisplatin. So far, pemetrexed combined with platinum is still the first-line chemotherapy standard protocol against MPM. Until 2016, the three-stage clinical MAPS study of chemotherapy-based combination with anti-vascular drugs confirmed that (7) standard pemetrexed combined with cisplatin alone can extend the median survival period from 16.1 to 18.8 months than that combined with bevacizumab. Currently, this joint protocol has been widely used as the first-line treatment toward non-operable MPM patients, and has been written into the NCCN guidelines (8). However, there is no standard treatment protocol of second-line chemotherapy for MPM currently, the recommended drugs mainly include gemcitabine, vinorelbine, or docetaxel (9-13), and these drugs can be used singly or combinedly. However, literature data have pointed out that as for the patients with better PS scores, the treatment protocol of combining two chemotherapy drugs can achieve better efficacies than single-drug chemotherapy. For example, Zucali et al. (12) reported that the protocol of gemcitabine combined with vinorelbine achieved the results of 2.8-month time to progress (TTP) and 10.9-month OS, and Tourkantonis et al. (13) reported that the protocol of gemcitabine combined with docetaxel achieved the results of 7-month TTP and 16.2-month OS.
Although the current clinical guidelines (8) recommend that pemetrexed combined with platinum, together with bevacizumab or not, can both be used as a first-line standard protocol for non-operable MPM patients. However, because bevacizumab needs patients to pay their own expense in China, so its first-line applications have been limited. Therefore, the first-line treatment toward all the patients enrolled into our study was pemetrexed combined with cisplatin while no bevacizumab was applied. In addition, some patients were detected the mutation of the epidermal growth factor receptor (EGFR) gene, and all the results showed wild-type. Because all the patients had better PS scores (0 to 1 point), we chose the second-line two-drug protocol. The reasons of selecting the protocol of gemcitabine combined with docetaxel were mainly the following aspects: (I) second-line chemotherapy still has no standard treatment protocol, and currently recommended drugs mainly include gemcitabine, vinorelbine, or docetaxel (9-13); (II) although certain literature has reported that vinorelbine has a certain effect as second-line chemotherapy for MPM (9-12), it still needs special treatment because of its vascular toxicity (14), so anti-vascular drugs such as bevacizumab can’t be considered shortly after deep vein catheterization (15); therefore, we did not choose vinorelbine; (III) not only Tourkantonis et al. (13) once reported that the protocol of gemcitabine combined with docetaxel achieved the results of 7-month TTP and 16.2-month OS, but also Ralli et al. (16) also reported in 2009 that the protocol of gemcitabine combined with docetaxel achieved the results of 7-month TTP and 15-month OS. Based on the above reasons, we chose gemcitabine combined with docetaxel as the second-line protocol. Our previous studies (17) have found that for the non-small cell lung cancer patients with EGFR mutation, the third-line bevacizumab combined with pemetrexed can significantly increase the efficacies of pemetrexed than pemetrexed alone, as well as can further extend the PFS and OS of late non-small cell lung cancer. Therefore, we believe that the combination of chemotherapy and anti-vascular therapy, even the second-line chemotherapy, can further improve the therapeutic effect of first-line pemetrexed combined with platinum in MPM patients who have not been applied bevacizumab yet. The results of this study show that the effective rates in groups A + GD and GD are 31.25% and 23.81%, respectively, and there is no statistical significance between the two groups. The disease-control rates are 75.00% and 52.38%, respectively (P=0.044), and there exists statistical significance between the two groups. The further observation of long-term efficacies reveals that the PFS in groups A + GD and GD were 4.0 and 5.4 months, respectively (χ2=4.615, P=0.032), and there was significant difference between the two groups. The OS was 11.3 and 13.6 months, respectively (χ2=4.484, P=0.028), and there was significant difference between the two groups. As for the adverse reactions in the two groups, the incidence of proteinuria, hypertension, and bleeding in group A + GD was significantly higher group GD, but no patient was withdrawn due to adverse reactions, consistent with the reports about the adverse effects of bevacizumab in treating MPM (7,18) or other solid tumors (19,20).
Conclusions
In summary, as for advanced MPM, the patients that progress after first-line standard pemetrexed combined with cisplatin and have better PS scores can be applied the second-line protocol of gemcitabine combined with docetaxel. Moreover, our study reveals for the first time that the addition of bevacizumab based on such treatment can further improve the disease control rate and prolong the PFS and OS.
Acknowledgments
Funding: This study was supported by Science and Technology Planning Project of Guangdong Province (2013B021800275, 2014A0-20212562, 2014A020212565) and Medical science and Technology Research Project of Guangdong Province (A2015409), China.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.01.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was conducted with approval from the Ethics Committee of Guangzhou Medical University [No. 2016(26)] and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang YY, Zhang H, Bai H, et al. Prognosis related clinical and molecular factors in malignant pleural mesothelioma. Zhonghua Jie He He Hu Xi Za Zhi 2013;36:162-8. [PubMed]
- Delgermaa V, Takahashi K, Park EK, et al. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ 2011;89:716-24, 724A-724C.
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [Crossref] [PubMed]
- Ceresoli GL, Zucali PA, Favaretto AG, et al. Phase II study of pemetrexed plus carboplatin in malignant pleural mesothelioma. J Clin Oncol 2006;24:1443-8. [Crossref] [PubMed]
- Castagneto B, Botta M, Aitini E, et al. Phase II study of pemetrexed in combination with carboplatin in patients with malignant pleural mesothelioma (MPM). Ann Oncol 2008;19:370-3. [Crossref] [PubMed]
- Katirtzoglou N, Gkiozos I, Makrilia N, et al. Carboplatin plus pemetrexed as first-line treatment of patients with malignant pleural mesothelioma: a phase II study. Clin Lung Cancer 2010;11:30-5. [Crossref] [PubMed]
- Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016;387:1405-14. [Crossref] [PubMed]
- Gradishar WJ, Anderson BO, Balassanian R, et al. NCCN Guidelines Insights: Breast Cancer, Version 1.2017. J Natl Compr Canc Netw 2017;15:433-51. [Crossref] [PubMed]
- Abdel-Rahman O, Kelany M. Systemic therapy options for malignant pleural mesothelioma beyond first-line therapy: a systematic review. Expert Rev Respir Med 2015;9:533-49. [Crossref] [PubMed]
- Zauderer MG, Kass SL, Woo K, et al. Vinorelbine and gemcitabine as second- or third-line therapy for malignant pleural mesothelioma. Lung Cancer 2014;84:271-4. [Crossref] [PubMed]
- Stebbing J, Powles T, McPherson K, et al. The efficacy and safety of weekly vinorelbine in relapsed malignant pleural mesothelioma. Lung Cancer 2009;63:94-7. [Crossref] [PubMed]
- Zucali PA, Ceresoli GL, Garassino I, et al. Gemcitabine and vinorelbine in pemetrexed-pretreated patients with malignant pleural mesothelioma. Cancer 2008;112:1555-61. [Crossref] [PubMed]
- Tourkantonis I, Makrilia N, Ralli M, et al. Phase II study of gemcitabine plus docetaxel as second-line treatment in malignant pleural mesothelioma: a single institution study. Am J Clin Oncol 2011;34:38-42. [Crossref] [PubMed]
- Rittenberg CN, Gralla RJ, Rehmeyer TA. Assessing and managing venous irritation associated with vinorelbine tartrate (Navelbine). Oncol Nurs Forum 1995;22:707-10. [PubMed]
- Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol 2009;6:465-77. [Crossref] [PubMed]
- Ralli M, Tourkantonis I, Makrilia N, et al. Docetaxel plus gemcitabine as first-line treatment in malignant pleural mesothelioma: a single institution phase II study. Anticancer Res 2009;29:3441-4. [PubMed]
- Zhou CZ, Qin YY, Xie ZH, et al. Efficacy of third-line pemetrexed monotherapy versus pemetrexed combination with bevacizumab in patients with advanced EGFR mutation-positive lung adenocarcinoma. Chin J Cancer Res 2014;26:705-10. [PubMed]
- Ceresoli GL, Zucali PA, Mencoboni M, et al. Phase II study of pemetrexed and carboplatin plus bevacizumab as first-line therapy in malignant pleural mesothelioma. Br J Cancer 2013;109:552-8. [Crossref] [PubMed]
- Hapani S, Sher A, Chu D, et al. Increased risk of serious hemorrhage with bevacizumab in cancer patients: a meta-analysis. Oncology 2010;79:27-38. [Crossref] [PubMed]
- Ranpura V, Pulipati B, Chu D, et al. Increased risk of high-grade hypertension with bevacizumab in cancer patients: a meta-analysis. Am J Hypertens 2010;23:460-8. [Crossref] [PubMed]


